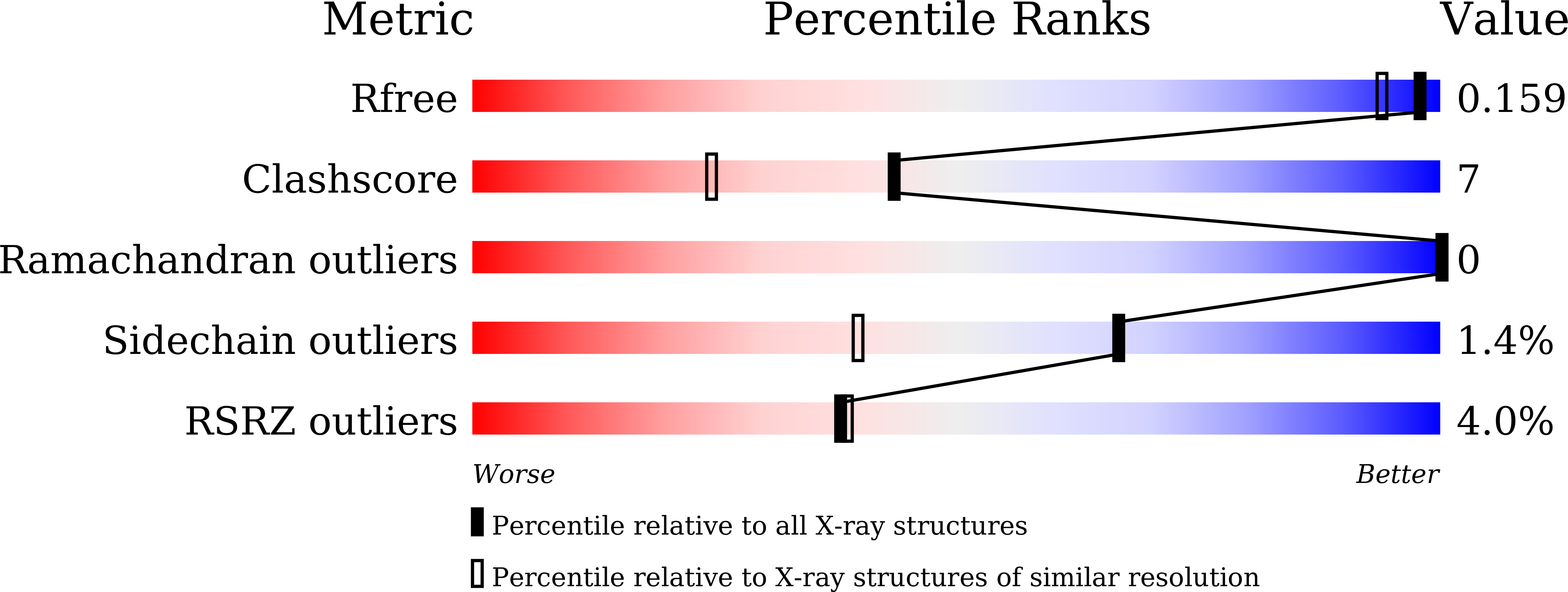
Interplay between the beta-lactam side chain and an active-site mobile loop of NDM-1 in penicillin hydrolysis as a potential target for mechanism-based inhibitor design.
Shi, X., Dai, Y., Lan, Z., Wang, S., Cui, L., Xiao, C., Zhao, K., Li, X., Liu, W., Zhang, Q.(2024) Int J Biol Macromol 262: 130041-130041
- PubMed: 38336327
- DOI: https://doi.org/10.1016/j.ijbiomac.2024.130041
- Primary Citation of Related Structures:
8GPC, 8GPD, 8GPE, 8I8F - PubMed Abstract:
Metallo-β-lactamases (MβLs) stand as significant resistant mechanism against β-lactam antibiotics in Gram-negative bacteria. The worldwide dissemination of New Delhi metallo-β-lactamases (NDMs) intensifies antimicrobial resistance, posing severe threats to human health due to the absence of inhibitors available in clinical therapy. L3, a flexible β-hairpin loop flanking the active site in MβLs, has been proven to wield influence over the reaction process by assuming a crucial role in substrate recognition and intermediate stabilization. In principle, it potentially retards product release from the enzyme, consequently reducing the overall turnover rate although the details regarding this aspect remain inadequately elucidated. In this study, we crystallized NDM-1 in complex with three penicillin substrates, conducted molecular dynamics simulations, and measured the steady-state kinetic parameters. These analyses consistently unveiled substantial disparities in their interactions with loop L3. We further synthesized a penicillin V derivative with increased hydrophobicity in the R1 side chain and co-crystallized it with NDM-1. Remarkably, this compound exhibited much stronger dynamic interplay with L3 during molecular dynamics simulation, showed much lower K m and k cat values, and demonstrated moderate inhibitory capacity to NDM-1 catalyzed meropenem hydrolysis. The data presented here may provide a strategic approach for designing mechanism-based MβL inhibitors focusing on structural elements external to the enzyme's active center.
Organizational Affiliation:
Department of Obstetrics and Gynecology, Daping Hospital, Army Medical University, Chongqing 400042, China.