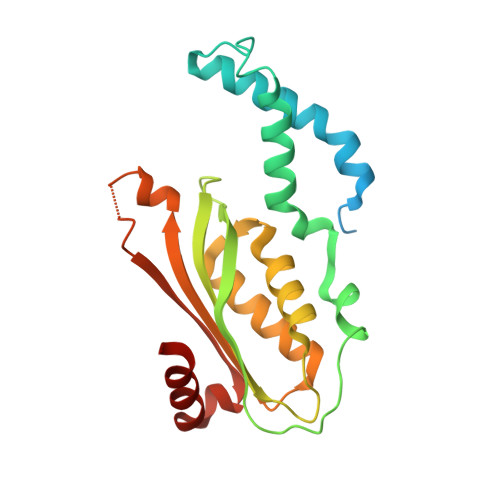
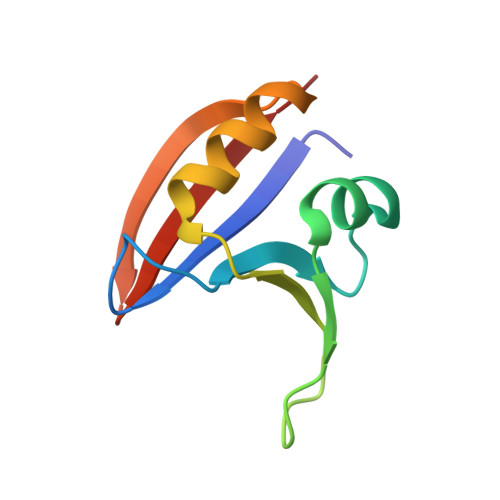
Crystal structure of the stimulatory complex of GTP cyclohydrolase I and its feedback regulatory protein GFRP.
Maita, N., Okada, K., Hatakeyama, K., Hakoshima, T.(2002) Proc Natl Acad Sci U S A 99: 1212-1217
- PubMed: 11818540
- DOI: https://doi.org/10.1073/pnas.022646999
- Primary Citation of Related Structures:
1IS7, 1IS8 - PubMed Abstract:
In the presence of phenylalanine, GTP cyclohydrolase I feedback regulatory protein (GFRP) forms a stimulatory 360-kDa complex with GTP cyclohydrolase I (GTPCHI), which is the rate-limiting enzyme in the biosynthesis of tetrahydrobiopterin. The crystal structure of the stimulatory complex reveals that the GTPCHI decamer is sandwiched by two GFRP homopentamers. Each GFRP pentamer forms a symmetrical five-membered ring similar to beta-propeller. Five phenylalanine molecules are buried inside each interface between GFRP and GTPCHI, thus enhancing the binding of these proteins. The complex structure suggests that phenylalanine-induced GTPCHI x GFRP complex formation enhances GTPCHI activity by locking the enzyme in the active state.
Organizational Affiliation:
Department of Molecular Biology, Nara Institute of Science and Technology, 8916-5 Takayama, Ikoma, Nara 630-0101, Japan.