Haloalkane dehalogenase LinB from Sphingomonas paucimobilis UT26: X-ray crystallographic studies of dehalogenation of brominated substrates
Streltsov, V.A., Prokop, Z., Damborsky, J., Nagata, Y., Oakley, A., Wilce, M.C.J.(2003) Biochemistry 42: 10104-10112
- PubMed: 12939138
- DOI: https://doi.org/10.1021/bi027280a
- Primary Citation of Related Structures:
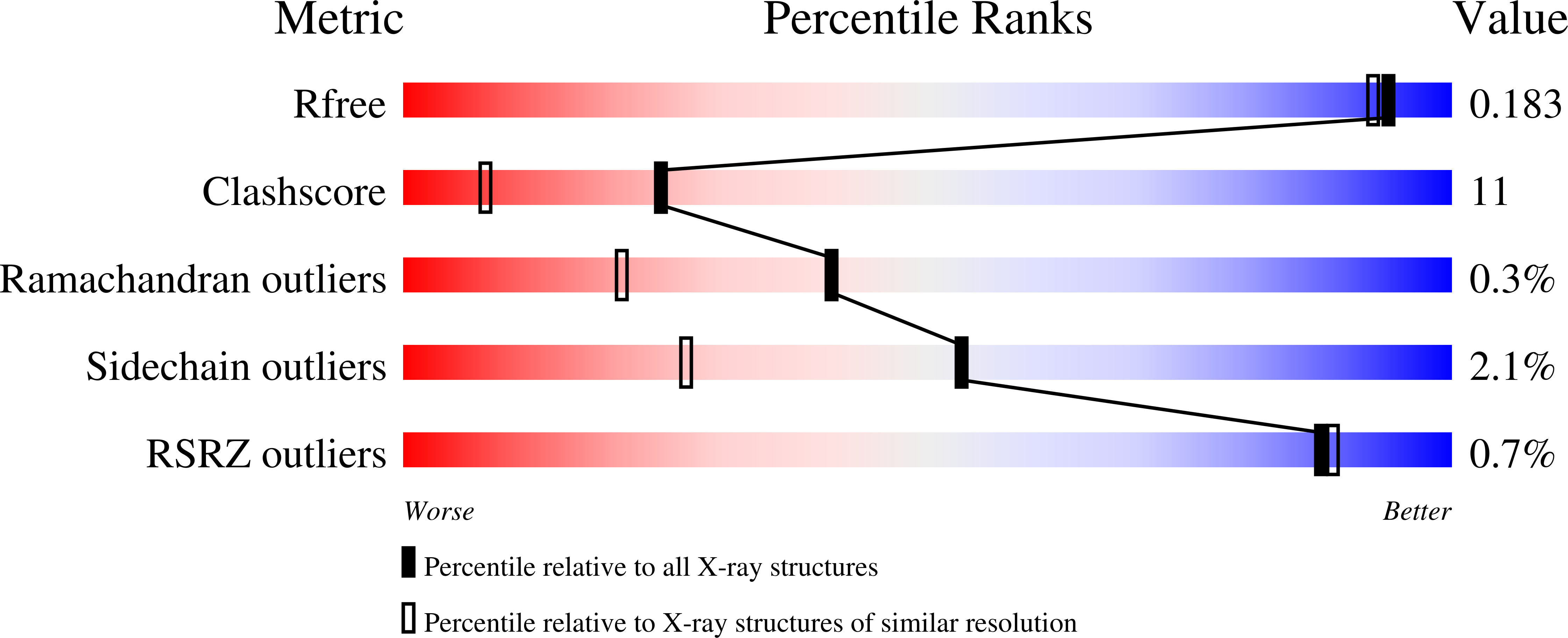
1IZ7, 1IZ8, 1K5P, 1K63, 1K6E - PubMed Abstract:
The haloalkane dehalogenases are detoxifying enzymes that convert a broad range of halogenated substrates to the corresponding alcohols. Complete crystal structures of haloalkane dehalogenase from Sphingomonas paucimobilis UT26 (LinB), and complexes of LinB with 1,2-propanediol/1-bromopropane-2-ol and 2-bromo-2-propene-1-ol, products of debromination of 1,2-dibromopropane and 2,3-dibromopropene, respectively, were determined from 1.8 A resolution X-ray diffraction data. Published structures of native LinB and its complex with 1,3-propanediol [Marek et al. (2000) Biochemistry 39, 14082-14086] were reexamined. The full and partial debromination of 1,2-dibromopropane and 2,3-dibromopropene, respectively, conformed to the observed general trend that the sp(3)-hybridized carbon is the predominant electrophilic site for the S(N)2 bimolecular nucleophilic substitution in dehalogenation reaction. The 2-bromo-2-propene-1-ol product of 2,3-dibromopropene dehalogenation in crystal was positively identified by the gas chromatography-mass spectroscopy (GC-MS) technique. The 1,2-propanediol and 1-bromopropane-2-ol products of 1,2-dibromopropane dehalogenation in crystal were also supported by the GC-MS identification. Comparison of native LinB with its complexes showed high flexibility of residues 136-157, in particular, Asp146 and Glu147, from the cap domain helices alpha(4) and alpha(5)('). Those residues were shifted mainly in direction toward the ligand molecules in the complex structures. It seems the cap domain moves nearer to the core squeezing substrate into the active center closer to the catalytic triad. This also leads to slight contraction of the whole complex structures. The flexibility detected by crystallographic analysis is in remarkable agreement with flexibility observed by molecular dynamic simulations.
Organizational Affiliation:
Crystallography Centre, School of Biomedical and Chemical Sciences, University of Western Australia, 35 Stirling Highway, Crawley 6009, Western Australia, Australia. [email protected]