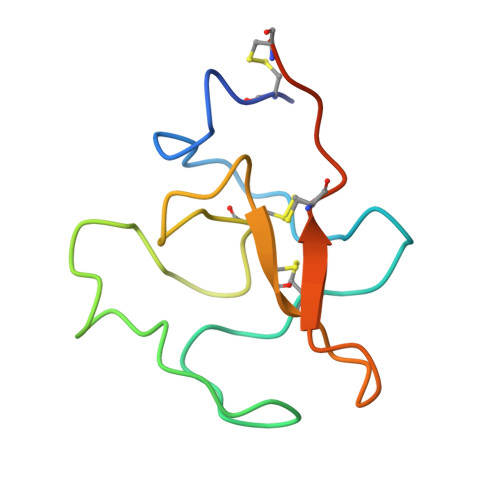
Structure of human plasminogen kringle 4 at 1.68 a and 277 K. A possible structural role of disordered residues.
Stec, B., Yamano, A., Whitlow, M., Teeter, M.M.(1997) Acta Crystallogr D Biol Crystallogr 53: 169-178
- PubMed: 15299951
- DOI: https://doi.org/10.1107/S0907444996012267
- Primary Citation of Related Structures:
1KRN - PubMed Abstract:
Despite considerable effort to elucidate the functional role of the kringle domains, relatively little is known about interactions with other protein domains. Most of the crystal structures describe the interactions at the kringle active site. This study suggests a novel way to interpret structural results such as disorder located away from an active site. The crystal structure of human plasminogen kringle 4 (PGK4) has been refined against 10-1.68 A resolution X-ray data (R(merge) = 3.7%) to the standard crystallographic R = 14.7% using the program X-PLOR. The crystals of PGK4 showed significant instability in cell dimensions (changes more than 1.5 A) even at 277 K. The refinement revealed structural details not observed before [Mulichak, Tulinsky & Ravichandran (1991). Biochemistry, 30, 10576-10588], such as clear density for additional side chains and more extensive disorder. Discrete disorder was detected for residues S73, S78, T80, S89, S91, S92, Ml12, S132, C138 and K142. Most of the disordered residues form two patches on the surface of the protein. This localized disorder suggests that these residues may play a role in quaternary interactions and possibly form an interface with the other domains of proteins that contain kringles, such as plasminogen. Although, an additional residue D65 was refined at the beginning of the sequence, still more residues near the peptide cleavage site must be disordered in the crystal.
Organizational Affiliation:
Department of Chemistry, Boston College, Chestnut Hill, MA 02167, USA. [email protected]