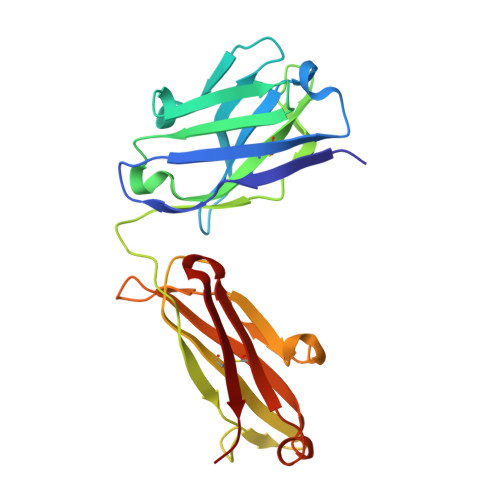
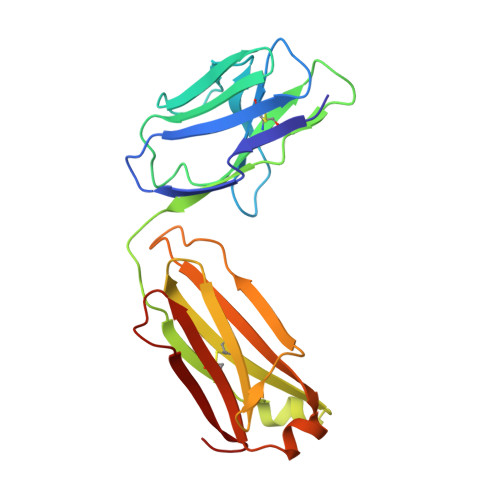
Crystal Structure of the alpha 1 beta 1 Integrin I Domain in Complex with an Antibody Fab Fragment
Karpusas, M., Ferrant, J., Weinreb, P., Carmillo, A., Taylor, F., Garber, E.(2003) J Mol Biol 327: 1031-1041
- PubMed: 12662928
- DOI: https://doi.org/10.1016/s0022-2836(03)00203-1
- Primary Citation of Related Structures:
1MHP - PubMed Abstract:
The alpha1beta1 (VLA-1) integrin is a cell-surface receptor for collagen and laminin and has been implicated in biological pathways involved in several pathological processes. These processes may be inhibited by the monoclonal antibody AQC2, which binds with high affinity to human alpha1beta1 integrin. To understand the structural basis of the inhibition we determined the crystal structure of the complex of a chimeric rat/human I domain of the alpha1beta1 integrin and the Fab fragment of humanized AQC2 antibody. The structure of the complex shows that the antibody blocks the collagen binding site of the I domain. An aspartate residue, from the CDR3 loop of the antibody heavy chain, coordinates the MIDAS metal ion in a manner similar to that of a glutamate residue from collagen. Substitution of the aspartate residue by alanine or arginine results in significant reduction of antibody binding affinity. Interestingly, although the mode of metal ion coordination resembles that of the open conformation, the I domain maintains an overall closed conformation previously observed only for unliganded I domains.
Organizational Affiliation:
Biogen, Inc., 14 Cambridge Center, Cambridge, MA 02142, USA. [email protected]