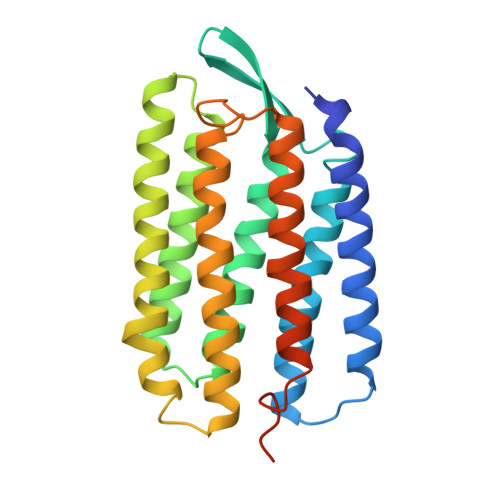
Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 A resolution.
Belrhali, H., Nollert, P., Royant, A., Menzel, C., Rosenbusch, J.P., Landau, E.M., Pebay-Peyroula, E.(1999) Structure 7: 909-917
- PubMed: 10467143
- DOI: https://doi.org/10.1016/s0969-2126(99)80118-x
- Primary Citation of Related Structures:
1QHJ - PubMed Abstract:
Bacteriorhodopsin (bR) from Halobacterium salinarum is a proton pump that converts the energy of light into a proton gradient that drives ATP synthesis. The protein comprises seven transmembrane helices and in vivo is organized into purple patches, in which bR and lipids form a crystalline two-dimensional array. Upon absorption of a photon, retinal, which is covalently bound to Lys216 via a Schiff base, is isomerized to a 13-cis,15-anti configuration. This initiates a sequence of events - the photocycle - during which a proton is transferred from the Schiff base to Asp85, followed by proton release into the extracellular medium and reprotonation from the cytoplasmic side. The structure of bR in the ground state was solved to 1.9 A resolution from non-twinned crystals grown in a lipidic cubic phase. The structure reveals eight well-ordered water molecules in the extracellular half of the putative proton translocation pathway. The water molecules form a continuous hydrogen-bond network from the Schiff-base nitrogen (Lys216) to Glu194 and Glu204 and includes residues Asp85, Asp212 and Arg82. This network is involved both in proton translocation occurring during the photocycle, as well as in stabilizing the structure of the ground state. Nine lipid phytanyl moieties could be modeled into the electron-density maps. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) analysis of single crystals demonstrated the presence of four different charged lipid species. The structure of protein, lipid and water molecules in the crystals represents the functional entity of bR in the purple membrane of the bacteria at atomic resolution. Proton translocation from the Schiff base to the extracellular medium is mediated by a hydrogen-bond network that involves charged residues and water molecules.
Organizational Affiliation:
ESRF, Grenoble, France.