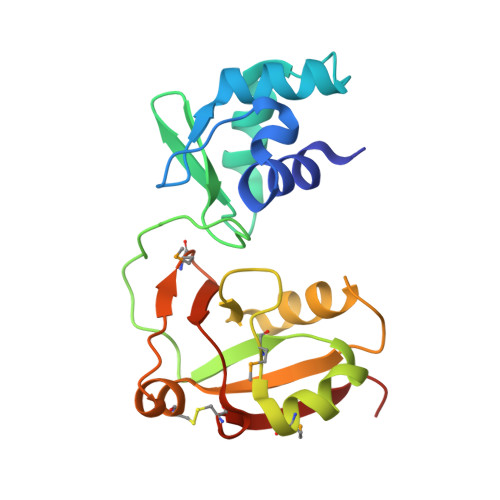
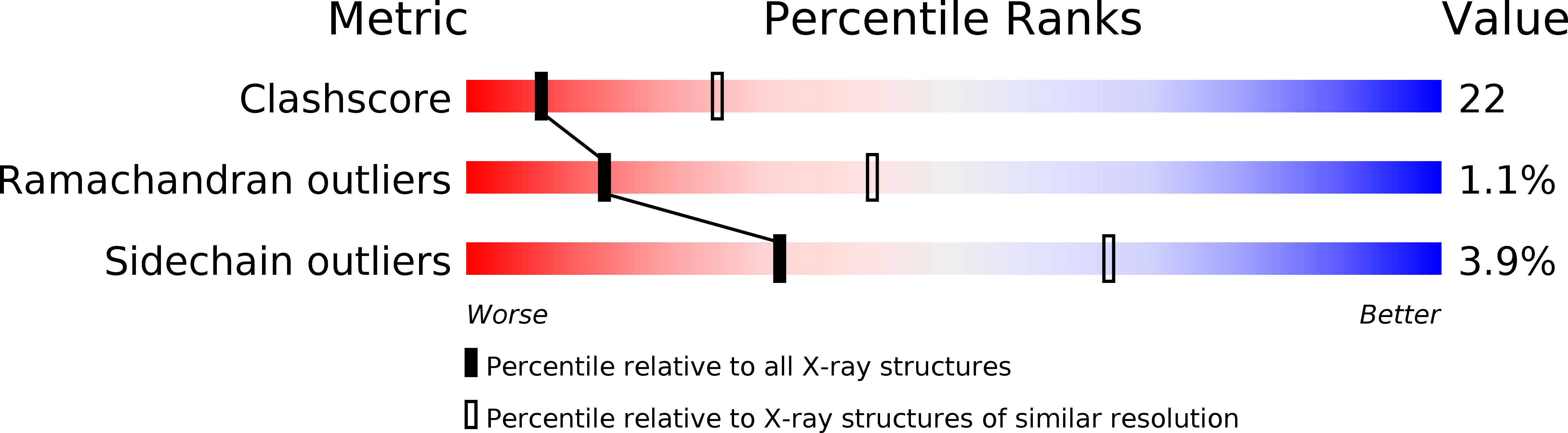Crystal Structure of the RNA 2'-Phosphotransferase from Aeropyrum pernix K1
Kato-Murayama, M., Bessho, Y., Shirouzu, M., Yokoyama, S.(2005) J Mol Biol 348: 295-305
- PubMed: 15811369
- DOI: https://doi.org/10.1016/j.jmb.2005.02.049
- Primary Citation of Related Structures:
1WFX - PubMed Abstract:
In the final step of tRNA splicing, the 2'-phosphotransferase catalyzes the transfer of the extra 2'-phosphate from the precursor-ligated tRNA to NAD. We have determined the crystal structure of the 2'-phosphotransferase protein from Aeropyrum pernix K1 at 2.8 Angstroms resolution. The structure of the 2'-phosphotransferase contains two globular domains (N and C-domains), which form a cleft in the center. The N-domain has the winged helix motif, a subfamily of the helix-turn-helix family, which is shared by many DNA-binding proteins. The C-domain of the 2'-phosphotransferase superimposes well on the NAD-binding fold of bacterial (diphtheria) toxins, which catalyze the transfer of ADP ribose from NAD to target proteins, indicating that the mode of NAD binding by the 2'-phosphotransferase could be similar to that of the bacterial toxins. The conserved basic residues are assembled at the periphery of the cleft and could participate in the enzyme contact with the sugar-phosphate backbones of tRNA. The modes by which the two functional domains recognize the two different substrates are clarified by the present crystal structure of the 2'-phosphotransferase.
Organizational Affiliation:
RIKEN Genomic Sciences Center, 1-7-22 Suehiro-cho, Tsurumi, Yokohama 230-0045, Japan.