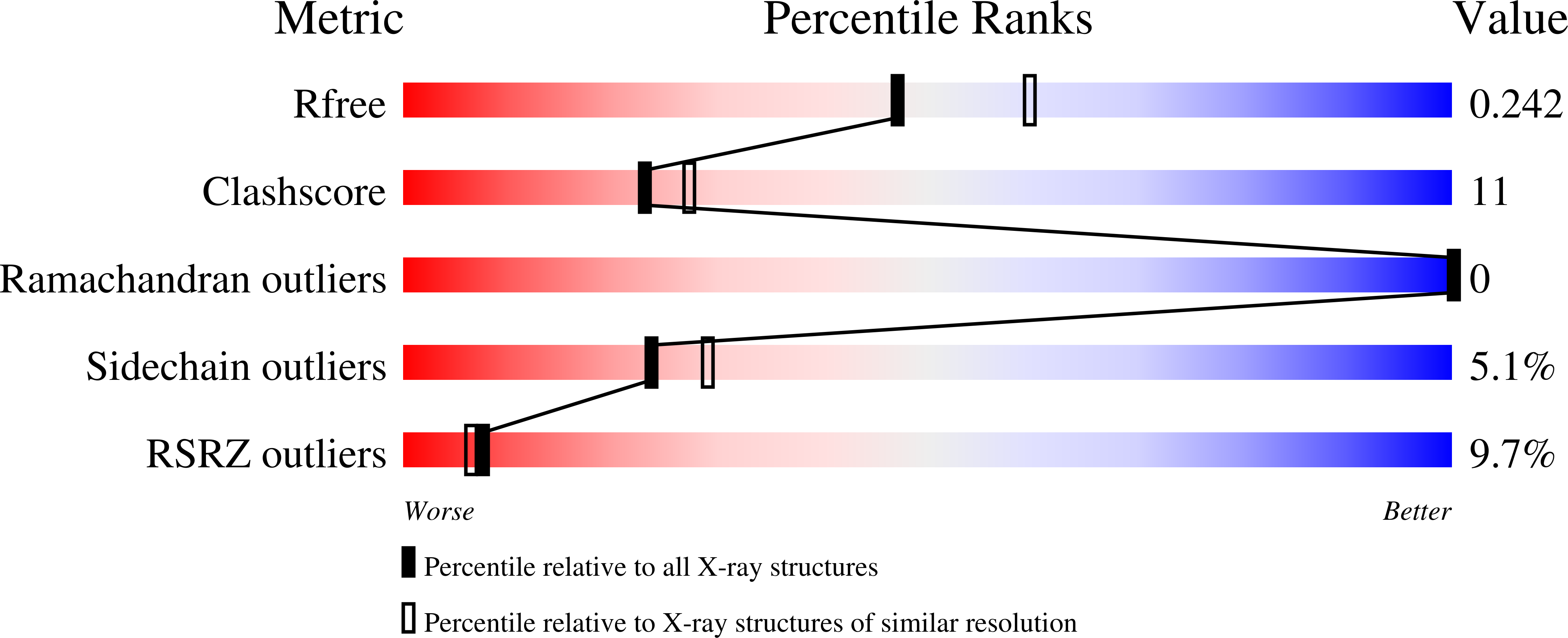
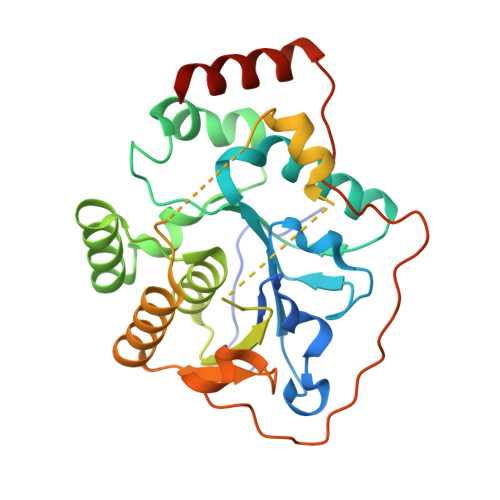
Structural Analysis of the alpha-2,3-Sialyltransferase Cst-I from Campylobacter jejuni in Apo and Substrate-Analogue Bound Forms.
Chiu, C.P., Lairson, L.L., Gilbert, M., Wakarchuk, W.W., Withers, S.G., Strynadka, N.C.(2007) Biochemistry 46: 7196-7204
- PubMed: 17518445
- DOI: https://doi.org/10.1021/bi602543d
- Primary Citation of Related Structures:
2P2V, 2P56 - PubMed Abstract:
Sialic acid is an essential sugar in biology that plays key roles in numerous cellular processes and interactions. The biosynthesis of sialylated glycoconjugates is catalyzed by five distinct families of sialyltransferases. In the last 25 years, there has been much research on the enzymes themselves, their genes, and their reaction products, but we still do not know the precise molecular mechanism of action for this class of glycosyltransferase. We previously reported the first detailed structural and kinetic characterization of Cst-II, a bifunctional sialyltransferase (CAZy GT-42) from the bacterium Campylobacter jejuni [Chiu et al. (2004) Nat. Struct. Mol. Biol. 11, 163-170]. This enzyme can use both Gal-beta-1,3/4-R and Neu5Ac-alpha-2,3-Gal-beta-1,3/4-R as acceptor sugars. A second sialyltransferase from this bacterium, Cst-I, has been shown to utilize solely Gal-beta-1,3/4-R as the acceptor sugar in its transferase reaction. We report here the structural and kinetic characterization of this monofunctional enzyme, which belongs to the same sialyltransferase family as Cst-II, in both apo and substrate bound form. Our structural data show that Cst-I adopts a similar GTA-type glycosyltransferase fold to that of the bifunctional Cst-II, with conservation of several key noncharged catalytic residues. Significant differences are found, however, between the two enzymes in the lid domain region, which is critical to the creation of the acceptor sugar binding site. Furthermore, molecular modeling of various acceptor sugars within the active sites of these enzymes provides significant new insights into the structural basis for substrate specificities within this biologically important enzyme class.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of British Columbia, 2350 Health Sciences Mall, Vancouver, British Columbia, V6T 1Z3, Canada.