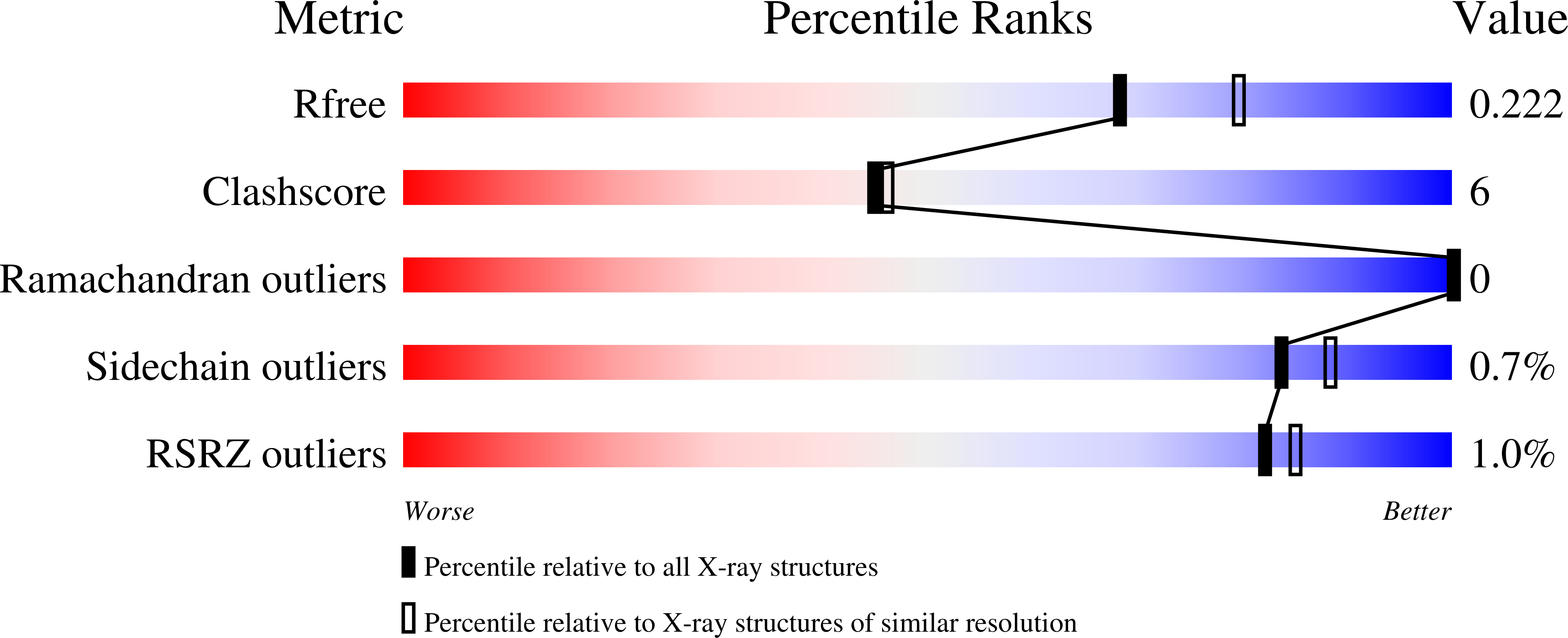
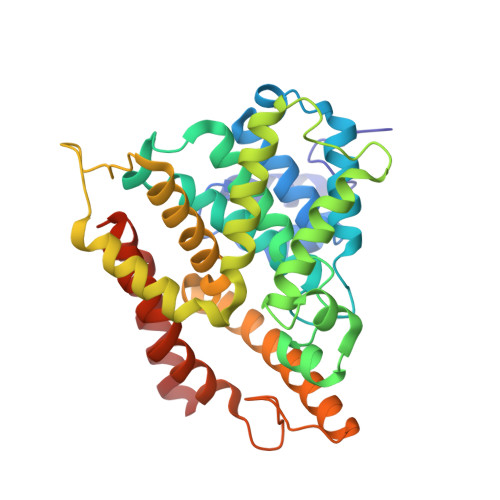
Structures of the four subfamilies of phosphodiesterase-4 provide insight into the selectivity of their inhibitors.
Wang, H., Peng, M.S., Chen, Y., Geng, J., Robinson, H., Houslay, M.D., Cai, J., Ke, H.(2007) Biochem J 408: 193-201
- PubMed: 17727341
- DOI: https://doi.org/10.1042/BJ20070970
- Primary Citation of Related Structures:
2QYK, 2QYL, 2QYM, 2QYN - PubMed Abstract:
PDE4 (phosphodiesterase-4)-selective inhibitors have attracted much attention as potential therapeutics for the treatment of both depression and major inflammatory diseases, but their practical application has been compromised by side effects. A possible cause for the side effects is that current PDE4-selective inhibitors similarly inhibit isoforms from all four PDE4 subfamilies. The development of PDE4 subfamily-selective inhibitors has been hampered by a lack of structural information. In the present study, we rectify this by providing the crystal structures of the catalytic domains of PDE4A, PDE4B and PDE4D in complex with the PDE4 inhibitor NVP {4-[8-(3-nitrophenyl)-[1,7]naphthyridin-6-yl]benzoic acid} as well as the unliganded PDE4C structure. NVP binds in the same conformation to the deep cAMP substrate pocket and interacts with the same residues in each instance. However, detailed structural comparison reveals significant conformational differences. Although the active sites of PDE4B and PDE4D are mostly comparable, PDE4A shows significant displacements of the residues next to the invariant glutamine residue that is critical for substrate and inhibitor binding. PDE4C appears to be more distal from other PDE4 subfamilies, with certain key residues being disordered. Our analyses provide the first structural basis for the development of PDE4 subfamily-selective inhibitors.
Organizational Affiliation:
Department of Biochemistry and Biophysics and Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, NC 27599-7260, USA.