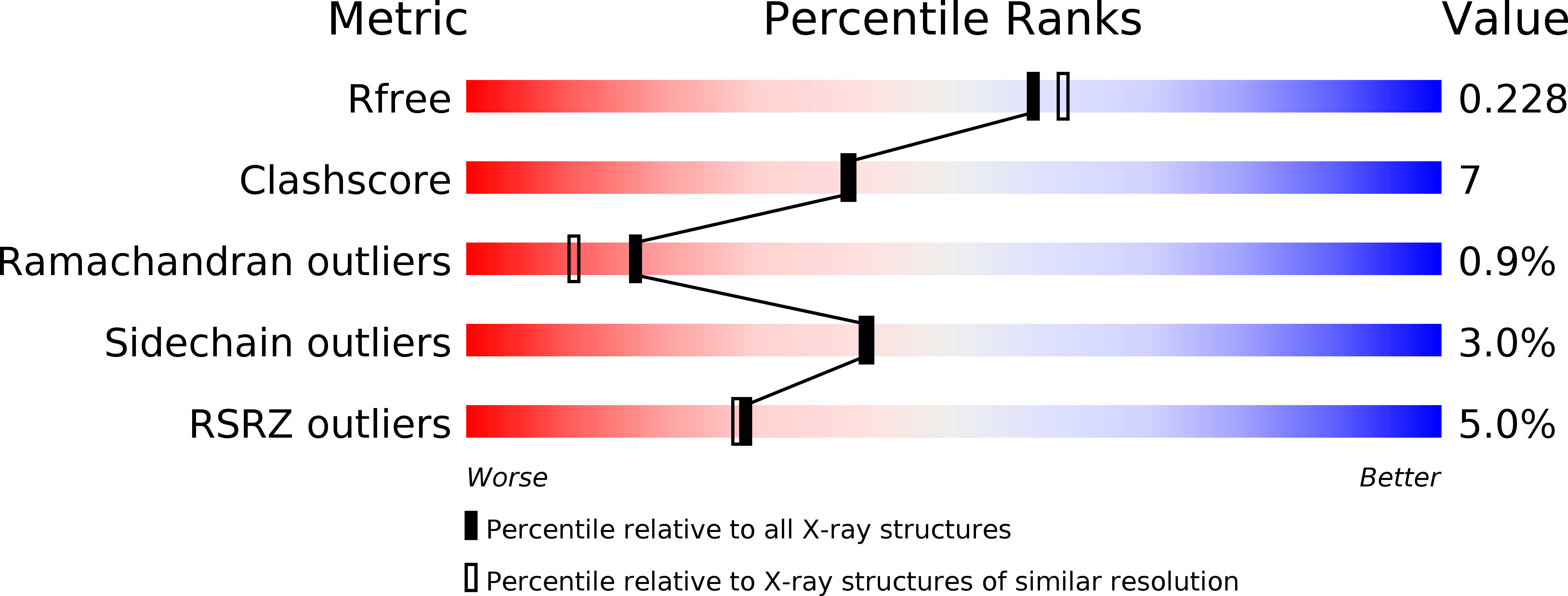
G Domain Dimerization Controls Dynamin'S Assembly-Stimulated Gtpase Activity.
Chappie, J.S., Acharya, S., Leonard, M., Schmid, S.L., Dyda, F.(2010) Nature 465: 435
- PubMed: 20428113
- DOI: https://doi.org/10.1038/nature09032
- Primary Citation of Related Structures:
2X2E, 2X2F - PubMed Abstract:
Dynamin is an atypical GTPase that catalyses membrane fission during clathrin-mediated endocytosis. The mechanisms of dynamin's basal and assembly-stimulated GTP hydrolysis are unknown, though both are indirectly influenced by the GTPase effector domain (GED). Here we present the 2.0 A resolution crystal structure of a human dynamin 1-derived minimal GTPase-GED fusion protein, which was dimeric in the presence of the transition state mimic GDP.AlF(4)(-).The structure reveals dynamin's catalytic machinery and explains how assembly-stimulated GTP hydrolysis is achieved through G domain dimerization. A sodium ion present in the active site suggests that dynamin uses a cation to compensate for the developing negative charge in the transition state in the absence of an arginine finger. Structural comparison to the rat dynamin G domain reveals key conformational changes that promote G domain dimerization and stimulated hydrolysis. The structure of the GTPase-GED fusion protein dimer provides insight into the mechanisms underlying dynamin-catalysed membrane fission.
Organizational Affiliation:
Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, Bethesda, Maryland 20892, USA.