Integration of Lead Optimization with Crystallography for a Membrane-Bound Ion Channel Target: Discovery of a New Class of Ampa Receptor Positive Allosteric Modulators.
Ward, S.E., Harries, M., Aldegheri, L., Austin, N.E., Ballantine, S., Ballini, E., Bradley, D.M., Bax, B.D., Clarke, B.P., Harris, A.J., Harrison, S.A., Melarange, R.A., Mookherjee, C., Mosley, J., Dal Negro, G., Oliosi, B., Smith, K.J., Thewlis, K.M., Woollard, P.M., Yusaf, S.P.(2011) J Med Chem 54: 78
- PubMed: 21128618
- DOI: https://doi.org/10.1021/jm100679e
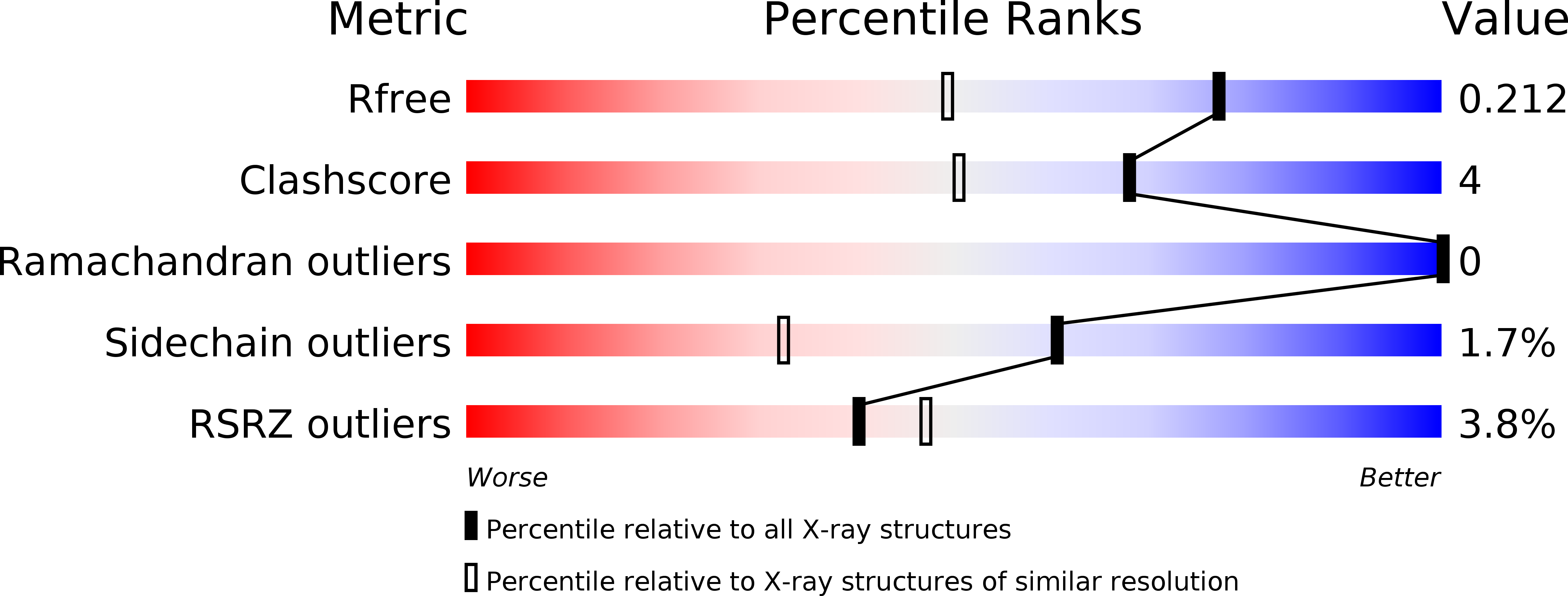
- Primary Citation of Related Structures:
2XX7, 2XX8, 2XX9, 2XXH, 2XXI - PubMed Abstract:
A novel series of AMPAR positive modulators is described that were identified by high throughput screening. The molecules of the series have been optimized from a high quality starting point hit to afford excellent developability, tolerability, and efficacy profiles, leading to identification of a clinical candidate. Unusually for an ion channel target, this optimization was integrated with regular generation of ligand-bound crystal structures and uncovered a novel chemotype with a unique and highly conserved mode of interaction via a trifluoromethyl group.
Organizational Affiliation:
School of Life Sciences, University of Sussex, Brighton, United Kingdom. [email protected]