Structural basis for the broad range substrate specificity of a novel mouse cytosolic sulfotransferase--mSULT1D1
Teramoto, T., Sakakibara, Y., Liu, M.-C., Suiko, M., Kimura, M., Kakuta, Y.(2009) Biochem Biophys Res Commun 379: 76-80
- PubMed: 19073143
- DOI: https://doi.org/10.1016/j.bbrc.2008.12.013
- Primary Citation of Related Structures:
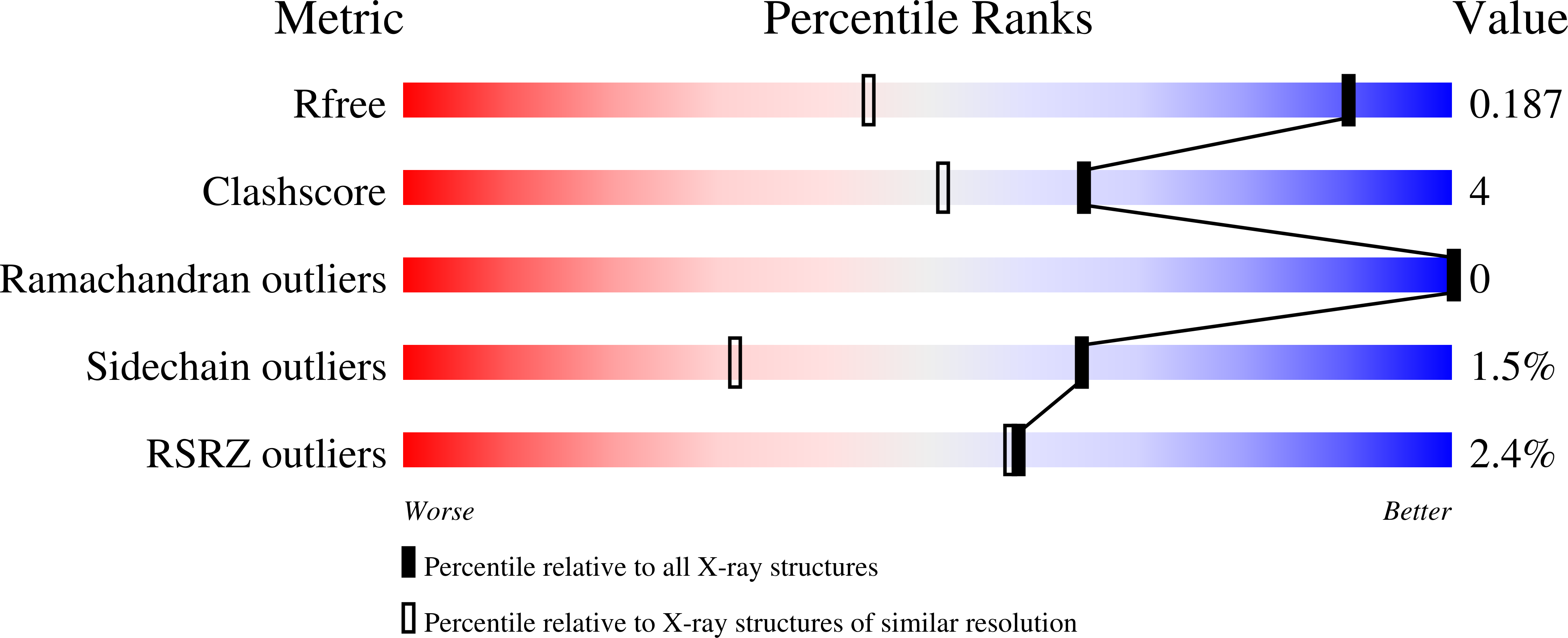
2ZVP, 2ZVQ - PubMed Abstract:
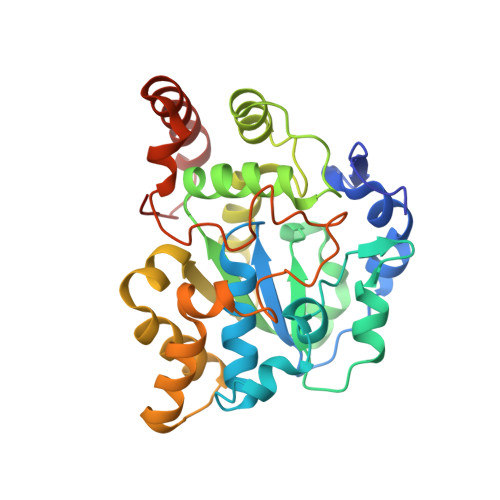
The mouse cytosolic sulfotransferase, mSULT1D1, catalyzes the sulfonation of a wide range of phenolic molecules including p-nitrophenol (pNP), alpha-naphthol (alphaNT), serotonin, as well as dopamine and its metabolites. To gain insight into the structural basis for its broad range substrate specificity, we solved two distinct ternary crystal structures of mSULT1D1, complexed with 3'-phosphoadenosine-5'-phosphate (PAP) plus pNP or PAP plus alphaNT. The structures revealed that the mSULT1D1 contains an L-shaped accepter-binding site which comprises 20 amino acid residues and four conserved water molecules. The shape of the accepter-binding site can be adjusted by conformational changes of two residues, Ile148 and Glu247, upon binding with respective substrates.
Organizational Affiliation:
Department of Systems Life Sciences, Laboratory of Structural Biology, Graduate School, Faculty of Agriculture, Kyushu University, Fukuoka, Japan.