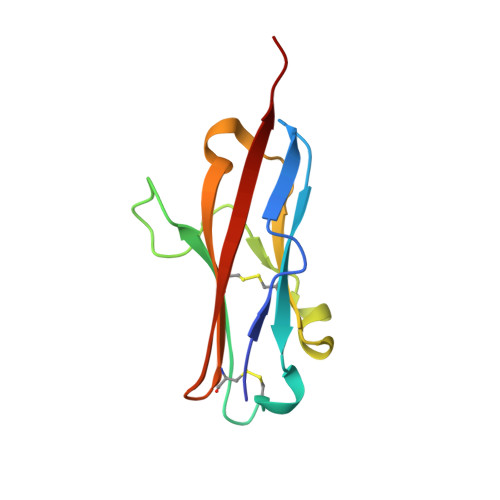
Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2.
Lazar-Molnar, E., Yan, Q., Cao, E., Ramagopal, U., Nathenson, S.G., Almo, S.C.(2008) Proc Natl Acad Sci U S A 105: 10483-10488
- PubMed: 18641123
- DOI: https://doi.org/10.1073/pnas.0804453105
- Primary Citation of Related Structures:
3BOV, 3BP5 - PubMed Abstract:
Programmed death-1 (PD-1) is a member of the CD28/B7 superfamily that delivers negative signals upon interaction with its two ligands, PD-L1 or PD-L2. The high-resolution crystal structure of the complex formed by the complete ectodomains of murine PD-1 and PD-L2 revealed a 1:1 receptor:ligand stoichiometry and displayed a binding interface and overall molecular organization distinct from that observed in the CTLA-4/B7 inhibitory complexes. Furthermore, our structure also provides insights into the association between PD-1 and PD-L1 and highlights differences in the interfaces formed by the two PD-1 ligands (PD-Ls) Mutagenesis studies confirmed the details of the proposed PD-1/PD-L binding interfaces and allowed for the design of a mutant PD-1 receptor with enhanced affinity. These studies define spatial and organizational constraints that control the localization and signaling of PD-1/PD-L complexes within the immunological synapse and provide a basis for manipulating the PD-1 pathways for immunotherapy.
Organizational Affiliation:
Department of Microbiology and Immunology, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA.