A concerted mechanism for berberine bridge enzyme
Winkler, A., Lyskowski, A., Riedl, S., Puhl, M., Kutchan, T.M., Macheroux, P., Gruber, K.(2008) Nat Chem Biol 4: 739-741
- PubMed: 18953357
- DOI: https://doi.org/10.1038/nchembio.123
- Primary Citation of Related Structures:
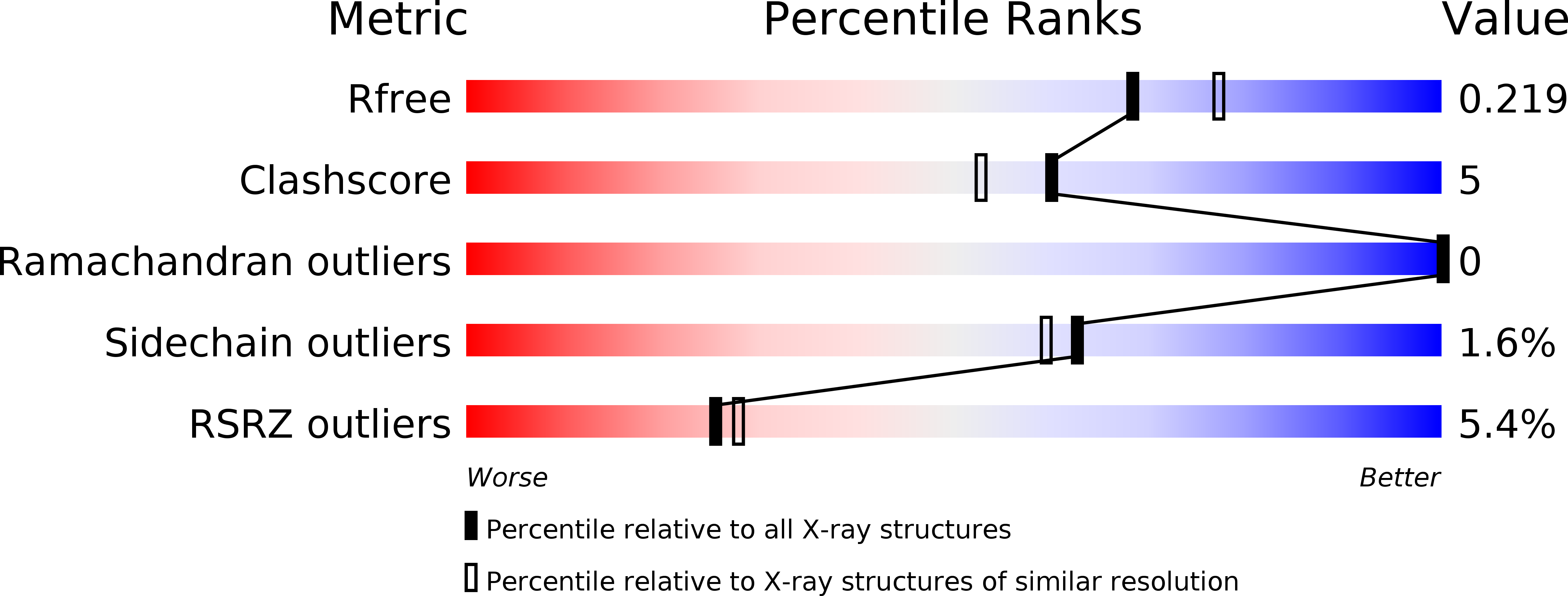
3D2D, 3D2H, 3D2J - PubMed Abstract:
Berberine bridge enzyme catalyzes the conversion of (S)-reticuline to (S)-scoulerine by formation of a carbon-carbon bond between the N-methyl group and the phenolic ring. We elucidated the structure of berberine bridge enzyme from Eschscholzia californica and determined the kinetic rates for three active site protein variants. Here we propose a catalytic mechanism combining base-catalyzed proton abstraction with concerted carbon-carbon coupling accompanied by hydride transfer from the N-methyl group to the N5 atom of the FAD cofactor.
Organizational Affiliation:
Institute of Biochemistry, Graz University of Technology, Petersgasse 12/II, 8010 Graz, Austria.