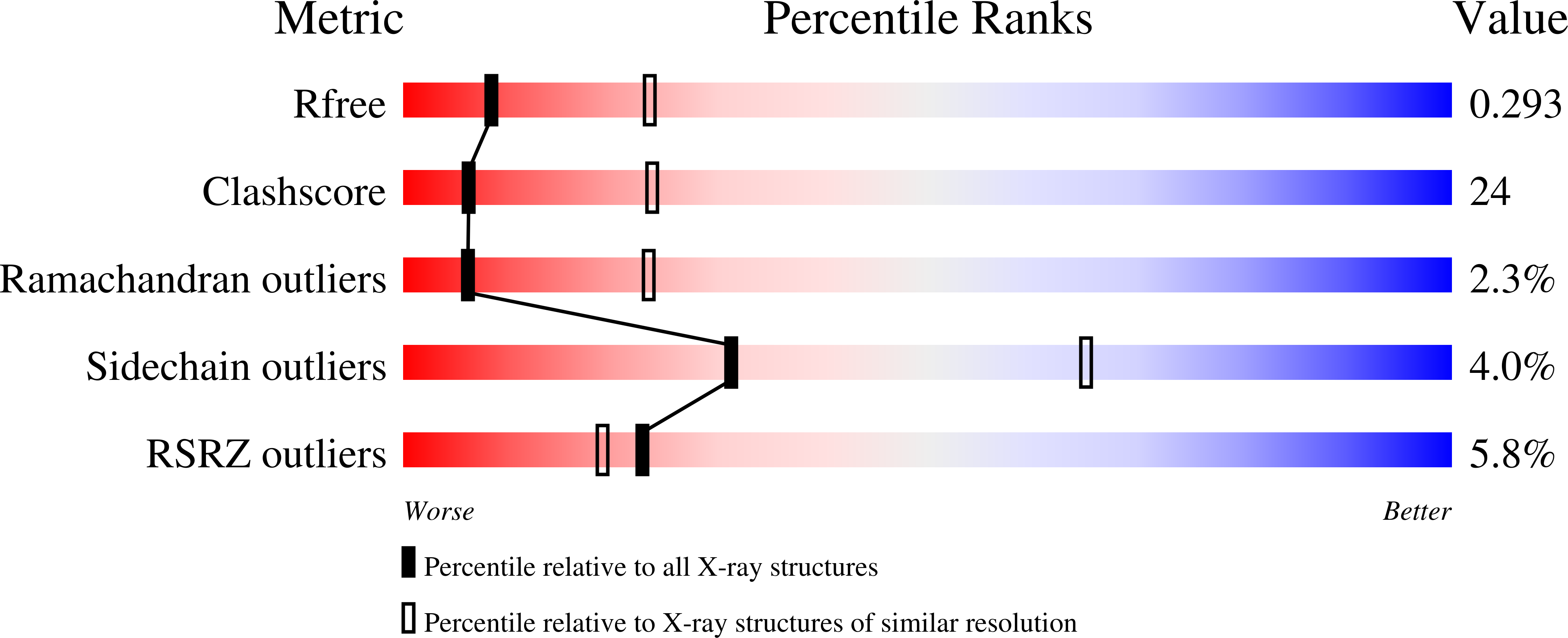
General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold.
Vincke, C., Loris, R., Saerens, D., Martinez-Rodriguez, S., Muyldermans, S., Conrath, K.(2008) J Biol Chem
- PubMed: 19010777
- DOI: https://doi.org/10.1074/jbc.M806889200
- Primary Citation of Related Structures:
3DWT, 3EAK, 3EBA - PubMed Abstract:
Nanobodies, single-domain antigen-binding fragments of camelid-specific heavy-chain only antibodies offer special advantages in therapy over classic antibody fragments because of their smaller size, robustness, and preference to target unique epitopes. A Nanobody differs from a human heavy chain variable domain in about ten amino acids spread all over its surface, four hallmark Nanobody-specific amino acids in the framework-2 region (positions 42, 49, 50, and 52), and a longer third antigen-binding loop (H3) folding over this area. For therapeutic applications the camelid-specific amino acid sequences in the framework have to be mutated to their human heavy chain variable domain equivalent, i.e. humanized. We performed this humanization exercise with Nanobodies of the subfamily that represents close to 80% of all dromedary-derived Nanobodies and investigated the effects on antigen affinity, solubility, expression yield, and stability. It is demonstrated that the humanization of Nanobody-specific residues outside framework-2 are neutral to the Nanobody properties. Surprisingly, the Glu-49 --> Gly and Arg-50 --> Leu humanization of hallmark amino acids generates a single domain that is more stable though probably less soluble. The other framework-2 substitutions, Phe-42 --> Val and Gly/Ala-52 --> Trp, are detrimental for antigen affinity, due to a repositioning of the H3 loop as shown by their crystal structures. These insights were used to identify a soluble, stable, well expressed universal humanized Nanobody scaffold that allows grafts of antigen-binding loops from other Nanobodies with transfer of the antigen specificity and affinity.
Organizational Affiliation:
Laboratory of Cellular and Molecular Immunology, Vrije Universiteit Brussel, Pleinlaan 2, Brussels B-1050; Department of Molecular and Cellular Interactions, Vlaams Instituut voor Biotechnologie, Brussels, Belgium.