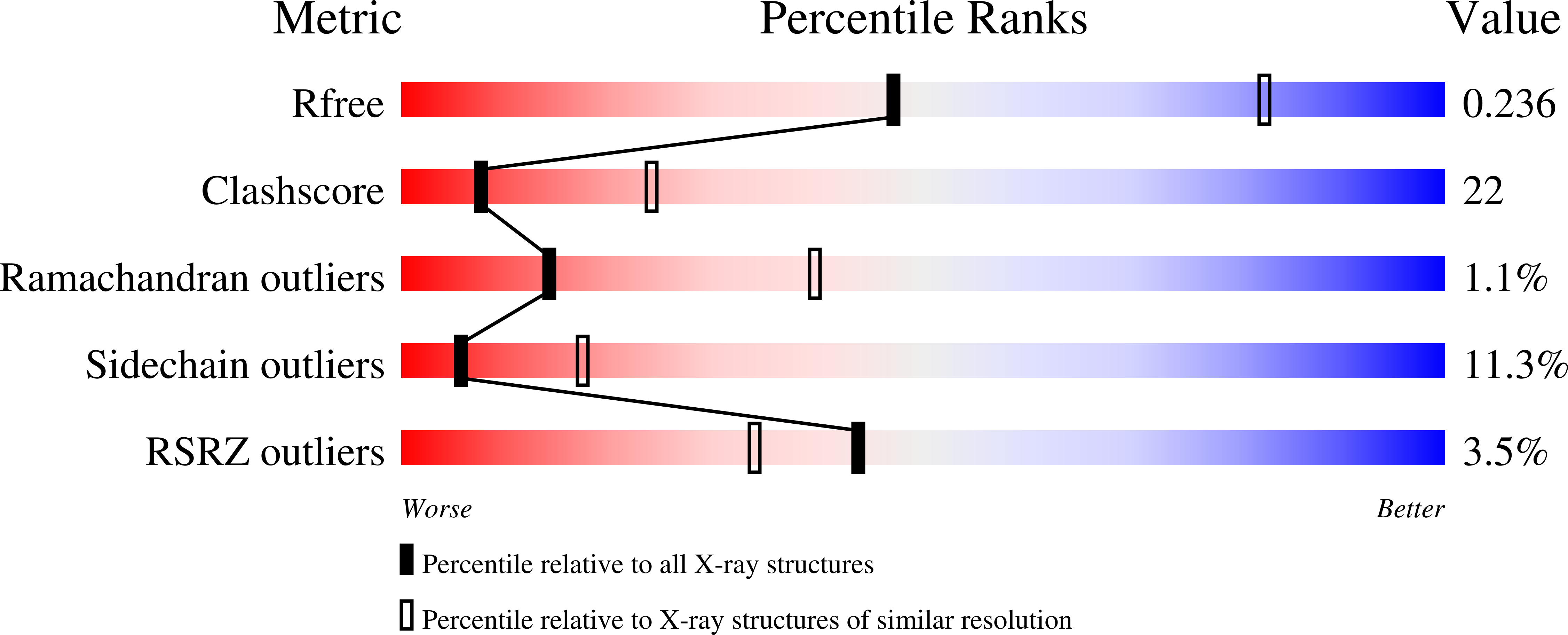
Correction for Han et al., "Biochemical and Structural Properties of Mouse Kynurenine Aminotransferase III".
Han, Q., Robinson, H., Cai, T., Tagle, D.A., Li, J.(2018) Mol Cell Biol 38
- PubMed: 29712768
- DOI: https://doi.org/10.1128/MCB.00099-18
- Primary Citation of Related Structures:
3E2F, 3E2Y, 3E2Z
Organizational Affiliation:
Department of Biochemistry, Virginia Tech, Blacksburg, Virginia 24061.