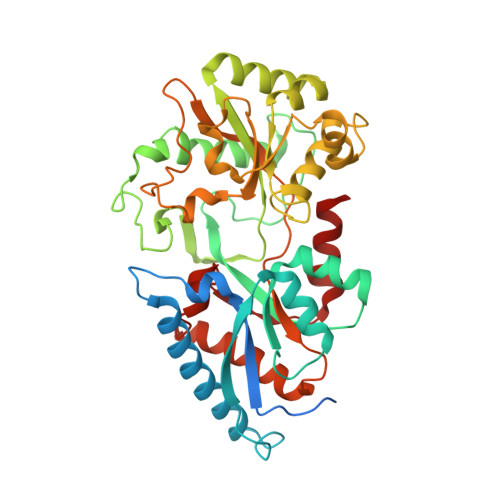
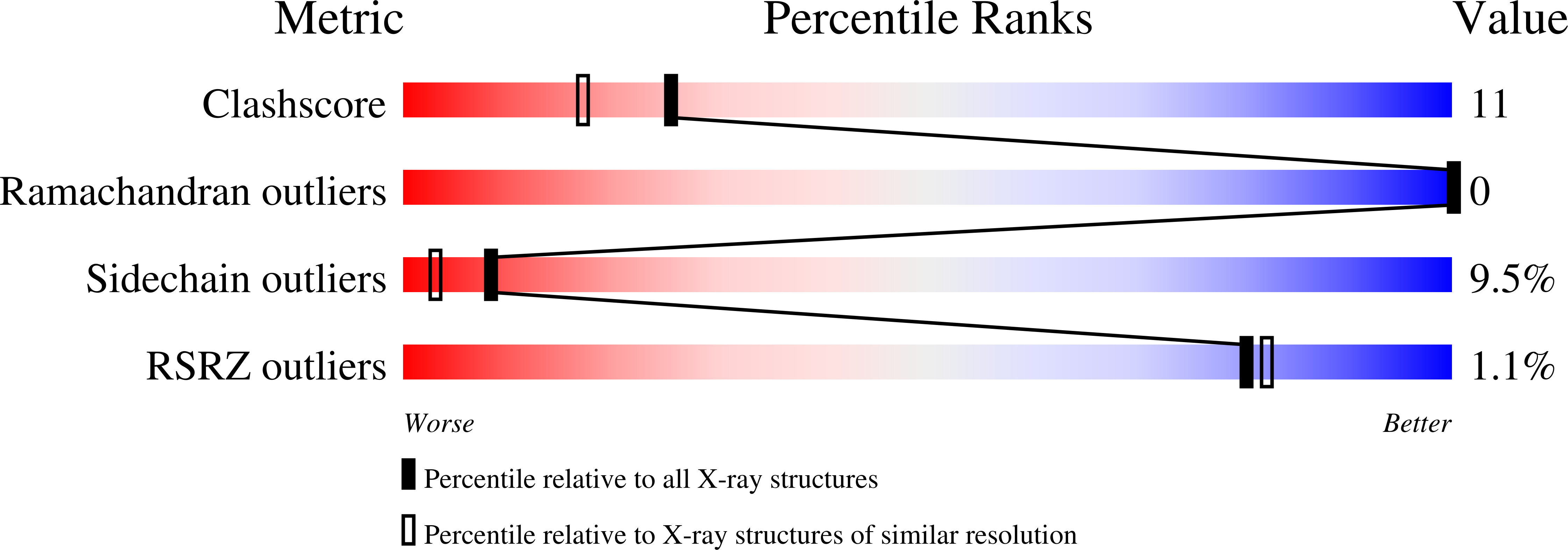Structure determination of the cancer-associated Mycoplasma hyorhinis protein Mh-p37.
Sippel, K.H., Robbins, A.H., Reutzel, R., Domsic, J., Boehlein, S.K., Govindasamy, L., Agbandje-McKenna, M., Rosser, C.J., McKenna, R.(2008) Acta Crystallogr D Biol Crystallogr 64: 1172-1178
- PubMed: 19020356
- DOI: https://doi.org/10.1107/S0907444908030175
- Primary Citation of Related Structures:
3E78, 3E79 - PubMed Abstract:
The crystal structure of the Mycoplasma hyorhinis protein Mh-p37 has been solved and refined to 1.9 A resolution. This is the first de novo structure to be determined using the recently described heavy-atom reagent [Beck et al. (2008), Acta Cryst. D64, 1179-1182] 5-amino-2,4,6-triiodoisophthalic acid (I3C), which contains three I atoms arranged in an equilateral triangle, by SIRAS methods. Data collection was performed in-house at room temperature. SHELXD and SHELXE were used to determine the I-atom positions and phase the native protein and PHENIX AutoBuild software was used to automatically fit the amino-acid sequence to the electron-density map. The structure was refined using SHELX97 to an R(cryst) of 18.6% and an R(free) of 24.0%. Mh-p37 is an alpha/beta protein with two well defined domains which are separated by a deep cleft. An unanticipated ligand bound in the center of the molecule at the base of the cleft has been modeled as thiamine pyrophosphate or vitamin B(1). Retrospective attempts to solve the crystal structure by Patterson search methods using either isomorphous or anomalous differences failed. Additionally, attempts to use proteins with the highest structural homology in the Protein Data Bank to phase the data by molecular replacement were unsuccessful, most likely in hindsight because of their poor structural agreement. Therefore, the I3C reagent offers an alternative, quick and inexpensive method for in-house phasing of de novo structures where other methods may not be successful.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Florida, Gainesville, FL 32610, USA.