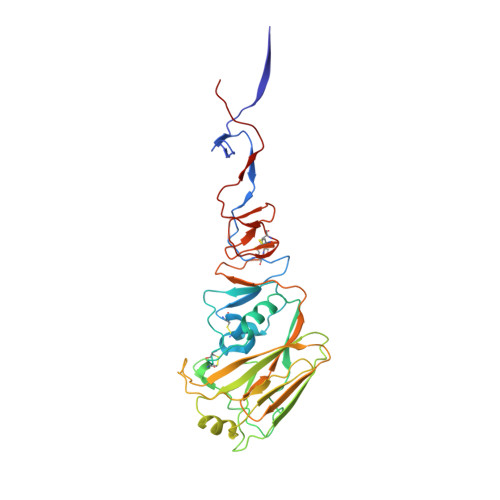
The hemagglutinin structure of an avian H1N1 influenza A virus
Lin, T., Wang, G., Li, A., Zhang, Q., Wu, C., Zhang, R., Cai, Q., Song, W., Yuen, K.-Y.(2009) Virology 392: 73-81
- PubMed: 19628241
- DOI: https://doi.org/10.1016/j.virol.2009.06.028
- Primary Citation of Related Structures:
3HTO, 3HTP, 3HTQ, 3HTT - PubMed Abstract:
The interaction between hemagglutinin (HA) and receptors is a kernel in the study of evolution and host adaptation of H1N1 influenza A viruses. The notion that the avian HA is associated with preferential specificity for receptors with Siaalpha2,3Gal glycosidic linkage over those with Siaalpha2,6Gal linkage is not all consistent with the available data on H1N1 viruses. By x-ray crystallography, the HA structure of an avian H1N1 influenza A virus, as well as its complexes with the receptor analogs, was determined. The structures revealed no preferential binding of avian receptor analogs over that of the human analog, suggesting that the HA/receptor binding might not be as stringent as is commonly believed in determining the host receptor preference for some subtypes of influenza viruses, such as the H1N1 viruses. The structure also showed difference in glycosylation despite the preservation of related sequences, which may partly contribute to the difference between structures of human and avian origin.
Organizational Affiliation:
School of Life Sciences, Xiamen University, Xiamen, PR China. [email protected]