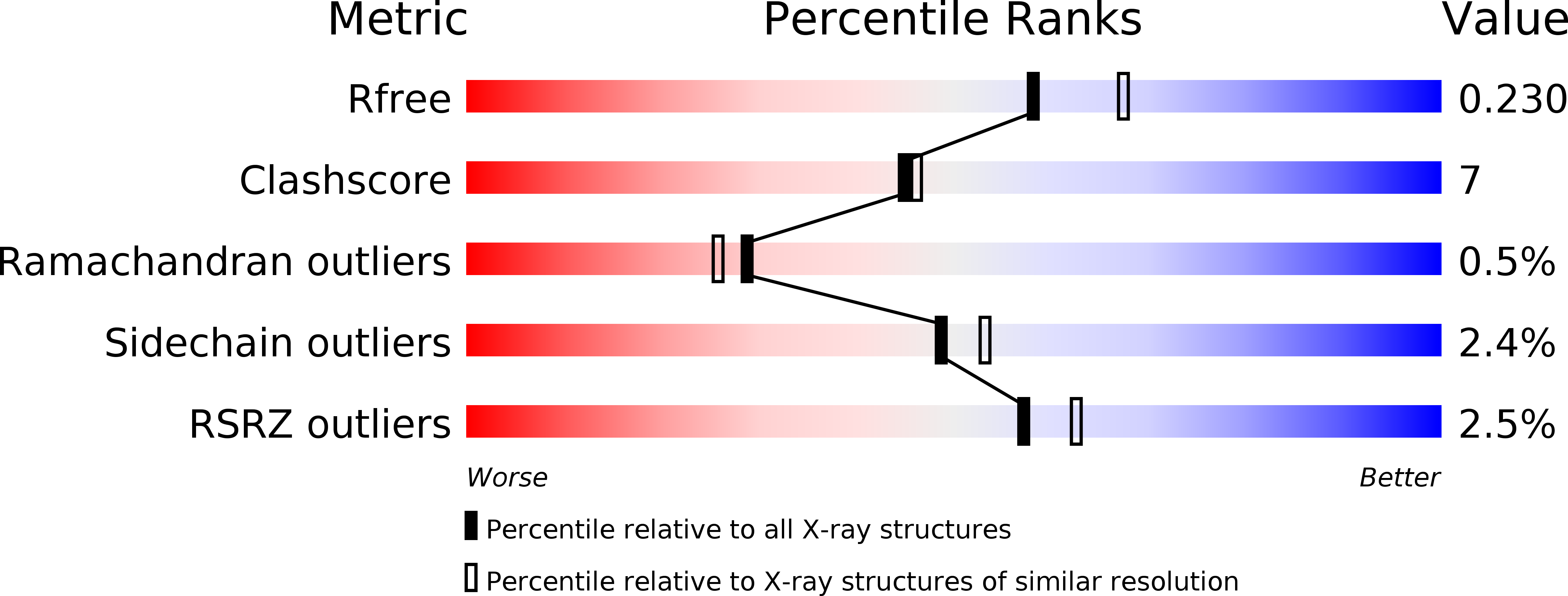
New glucosidase inhibitors from an ayurvedic herbal treatment for type 2 diabetes: structures and inhibition of human intestinal maltase-glucoamylase with compounds from Salacia reticulata.
Sim, L., Jayakanthan, K., Mohan, S., Nasi, R., Johnston, B.D., Pinto, B.M., Rose, D.R.(2010) Biochemistry 49: 443-451
- PubMed: 20039683
- DOI: https://doi.org/10.1021/bi9016457
- Primary Citation of Related Structures:
3L4T, 3L4U, 3L4V, 3L4W, 3L4X, 3L4Y, 3L4Z - PubMed Abstract:
An approach to controlling blood glucose levels in individuals with type 2 diabetes is to target alpha-amylases and intestinal glucosidases using alpha-glucosidase inhibitors acarbose and miglitol. One of the intestinal glucosidases targeted is the N-terminal catalytic domain of maltase-glucoamylase (ntMGAM), one of the four intestinal glycoside hydrolase 31 enzyme activities responsible for the hydrolysis of terminal starch products into glucose. Here we present the X-ray crystallographic studies of ntMGAM in complex with a new class of alpha-glucosidase inhibitors derived from natural extracts of Salacia reticulata, a plant used traditionally in Ayuverdic medicine for the treatment of type 2 diabetes. Included in these extracts are the active compounds salacinol, kotalanol, and de-O-sulfonated kotalanol. This study reveals that de-O-sulfonated kotalanol is the most potent ntMGAM inhibitor reported to date (K(i) = 0.03 microM), some 2000-fold better than the compounds currently used in the clinic, and highlights the potential of the salacinol class of inhibitors as future drug candidates.
Organizational Affiliation:
Ontario Cancer Institute and Department of Medical Biophysics, University of Toronto, 101 College Street, Toronto, ON, M5G 1L7 Canada.