Structural basis of the inhibition of class C acid phosphatases by adenosine 5'-phosphorothioate.
Singh, H., Reilly, T.J., Tanner, J.J.(2011) FEBS J 278: 4374-4381
- PubMed: 21933344
- DOI: https://doi.org/10.1111/j.1742-4658.2011.08360.x
- Primary Citation of Related Structures:
3OCZ, 3SF0 - PubMed Abstract:
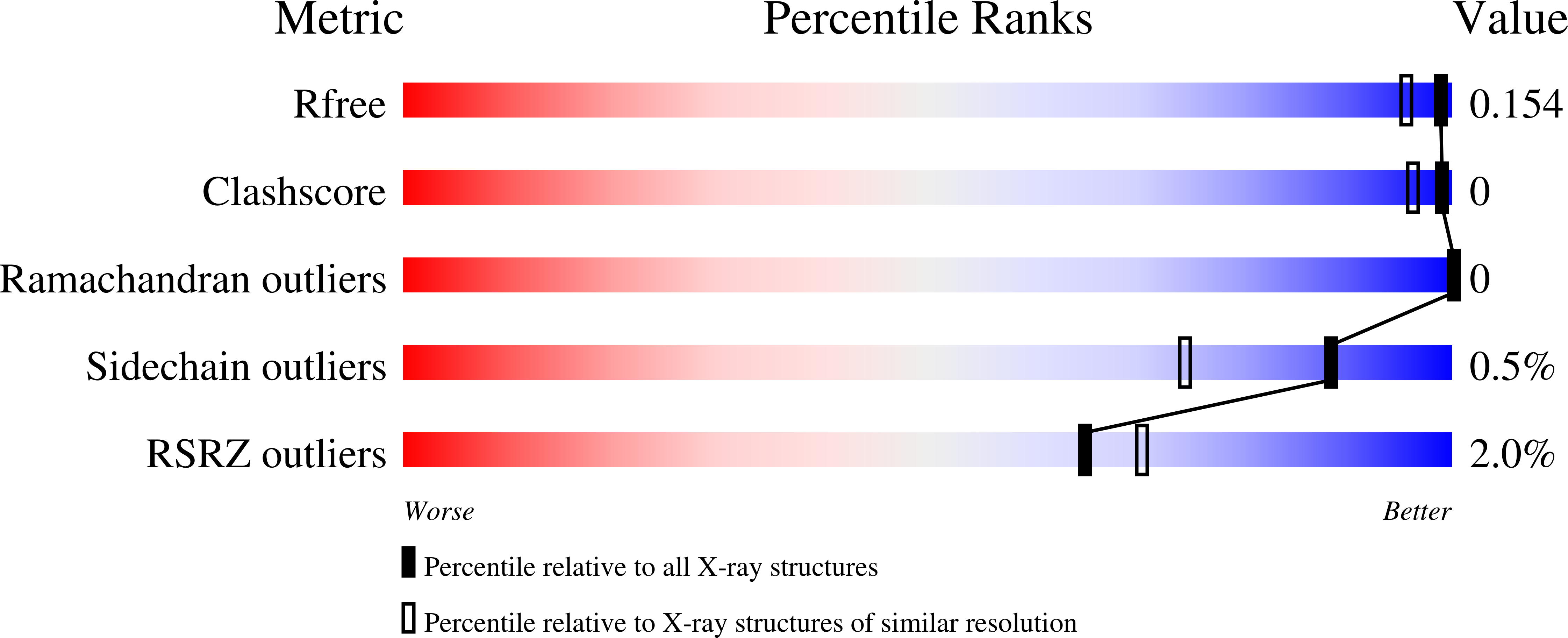
The inhibition of phosphatases by adenosine 5'-phosphorothioate (AMPS) was first reported in the late 1960s; however, the structural basis for the inhibition has remained unknown. Here, it is shown that AMPS is a submicromolar inhibitor of class C acid phosphatases, a group of bacterial outer membrane enzymes belonging to the haloacid dehalogenase structural superfamily. Furthermore, the 1.35-Å resolution crystal structure of the inhibited recombinant Haemophilus influenzae class C acid phosphatase was determined; this is the first structure of a phosphatase complexed with AMPS. The conformation of AMPS is identical to that of the substrate 5'-AMP, except that steric factors force a rotation of the thiophosphoryl out of the normal phosphoryl-binding pocket. This conformation is catalytically nonproductive, because the P atom is not positioned optimally for nucleophilic attack by Asp64, and the O atom of the scissile O-P bond is too far from the Asp (Asp66) that protonates the leaving group. The structure of 5'-AMP complexed with the Asp64→Asn mutant enzyme was also determined at 1.35-Å resolution. This mutation induces the substrate to adopt the same nonproductive binding mode that is observed in the AMPS complex. In this case, electrostatic considerations, rather than steric factors, underlie the movement of the phosphoryl. The structures not only provide an explanation for the inhibition by AMPS, but also highlight the precise steric and electrostatic requirements of phosphoryl recognition by class C acid phosphatases. Moreover, the structure of the Asp64→Asn mutant illustrates how a seemingly innocuous mutation can cause an unexpected structural change.
Organizational Affiliation:
Department of Chemistry, University of Missouri-Columbia, Columbia, MO 65211, USA.