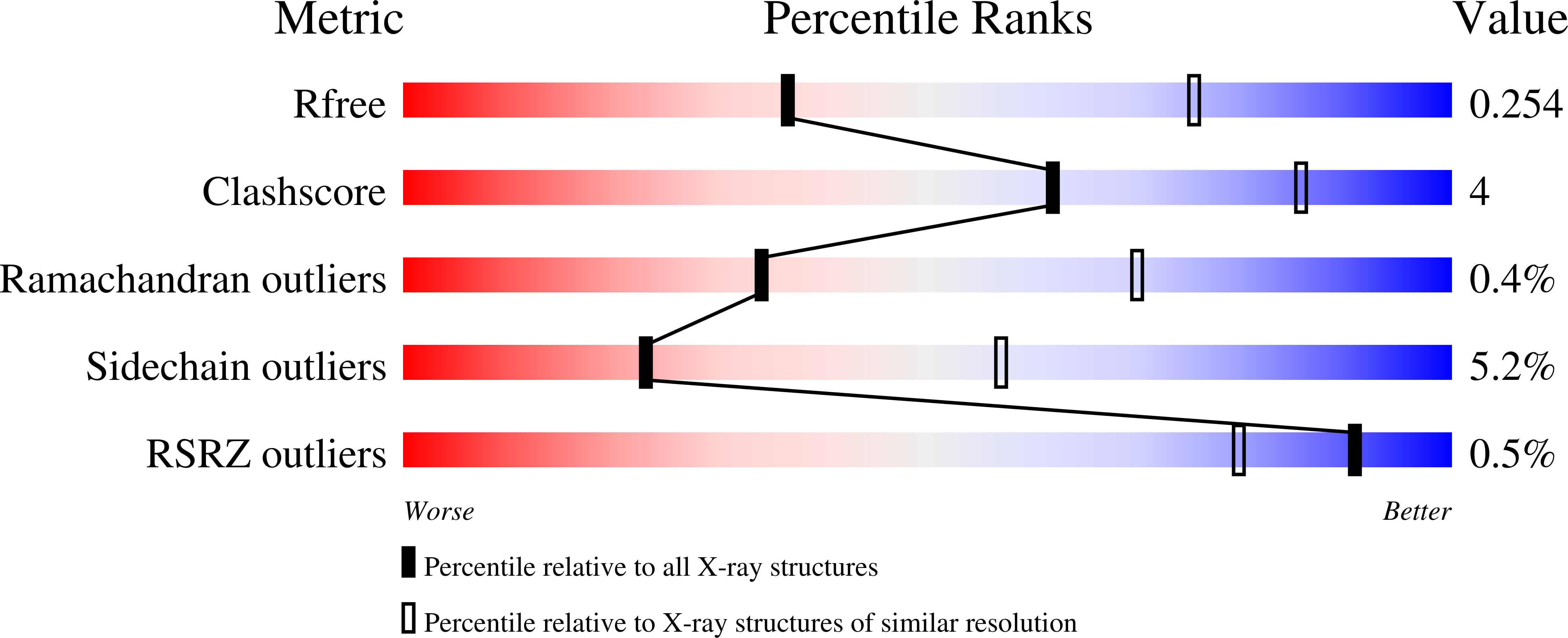
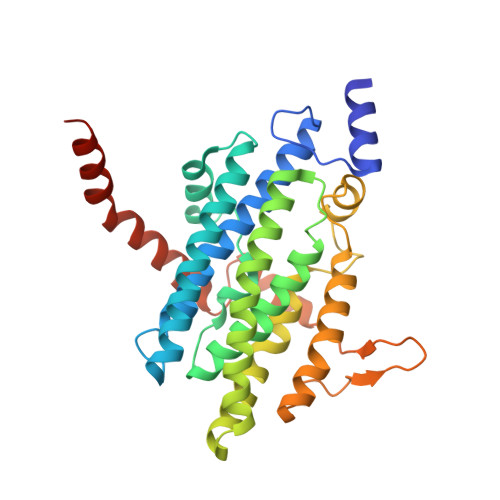
Structural and Functional Studies of the Escherichia coli Phenylacetyl-CoA Monooxygenase Complex.
Grishin, A.M., Ajamian, E., Tao, L., Zhang, L., Menard, R., Cygler, M.(2011) J Biol Chem 286: 10735-10743
- PubMed: 21247899
- DOI: https://doi.org/10.1074/jbc.M110.194423
- Primary Citation of Related Structures:
3PVR, 3PVT, 3PVY, 3PW1, 3PW8, 3PWQ - PubMed Abstract:
The utilization of phenylacetic acid (PA) in Escherichia coli occurs through a hybrid pathway that shows features of both aerobic and anaerobic metabolism. Oxygenation of the aromatic ring is performed by a multisubunit phenylacetyl-coenzyme A oxygenase complex that shares remote homology of two subunits to well studied bacterial multicomponent monooxygenases and was postulated to form a new bacterial multicomponent monooxygenase subfamily. We expressed the subunits PaaA, B, C, D, and E of the PA-CoA oxygenase and showed that PaaABC, PaaAC, and PaaBC form stable subcomplexes that can be purified. In vitro reconstitution of the oxygenase subunits showed that each of the PaaA, B, C, and E subunits are necessary for catalysis, whereas PaaD is not essential. We have determined the crystal structure of the PaaAC complex in a ligand-free form and with several CoA derivatives. We conclude that PaaAC forms a catalytic core with a monooxygenase fold with PaaA being the catalytic α subunit and PaaC, the structural β subunit. PaaAC forms heterotetramers that are organized very differently from other known multisubunit monooxygenases and lacks their conservative network of hydrogen bonds between the di-iron center and protein surface, suggesting different association with the reductase and different mechanisms of electron transport. The PaaA structure shows adaptation of the common access route to the active site for binding a CoA-bound substrate. The enzyme-substrate complex shows the orientation of the aromatic ring, which is poised for oxygenation at the ortho-position, in accordance with the expected chemistry. The PA-CoA oxygenase complex serves as a paradigm for the new subfamily multicomponent monooxygenases comprising several hundred homologs.
Organizational Affiliation:
Department of Biochemistry, McGill University, Montreal, Quebec H3G 1Y6, Canada.