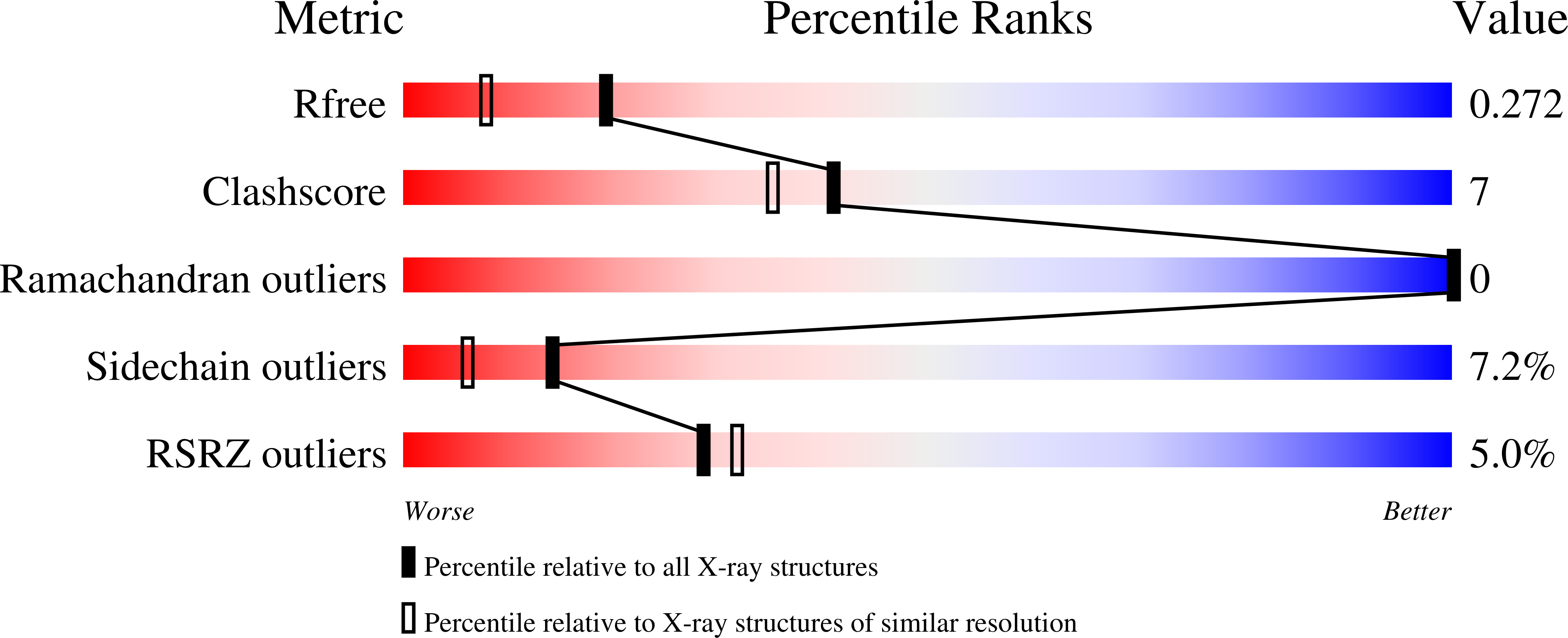
Structural, Kinetic, and Pharmacodynamic Mechanisms of D-Amino Acid Oxidase Inhibition by Small Molecules.
Hopkins, S.C., Heffernan, M.L.R., Saraswat, L.D., Bowen, C.A., Melnick, L., Hardy, L.W., Orsini, M.A., Allen, M.S., Koch, P., Spear, K.L., Foglesong, R.J., Soukri, M., Chytil, M., Fang, Q.K., Jones, S.W., Varney, M.A., Panatier, A., Oliet, S.H.R., Pollegioni, L., Piubelli, L., Molla, G., Nardini, M., Large, T.H.(2013) J Med Chem 56: 3710
- PubMed: 23631755
- DOI: https://doi.org/10.1021/jm4002583
- Primary Citation of Related Structures:
3ZNN, 3ZNO, 3ZNP, 3ZNQ - PubMed Abstract:
We characterized the mechanism and pharmacodynamics of five structurally distinct inhibitors of d-amino acid oxidase. All inhibitors bound the oxidized form of human enzyme with affinity slightly higher than that of benzoate (Kd ≈ 2-4 μM). Stopped-flow experiments showed that pyrrole-based inhibitors possessed high affinity (Kd ≈ 100-200 nM) and slow release kinetics (k < 0.01 s(-1)) in the presence of substrate, while inhibitors with pendent aromatic groups altered conformations of the active site lid, as evidenced by X-ray crystallography, and showed slower kinetics of association. Rigid bioisosteres of benzoic acid induced a closed-lid conformation, had slower release in the presence of substrate, and were more potent than benzoate. Steady-state d-serine concentrations were described in a PK/PD model, and competition for d-serine sites on NMDA receptors was demonstrated in vivo. DAAO inhibition increased the spatiotemporal influence of glial-derived d-serine, suggesting localized effects on neuronal circuits where DAAO can exert a neuromodulatory role.
Organizational Affiliation:
Sunovion Pharmaceuticals Inc., Marlborough, Massachusetts 01752, United States. [email protected]