Designing allosteric control into enzymes by chemical rescue of structure.
Deckert, K., Budiardjo, S.J., Brunner, L.C., Lovell, S., Karanicolas, J.(2012) J Am Chem Soc 134: 10055-10060
- PubMed: 22655749
- DOI: https://doi.org/10.1021/ja301409g
- Primary Citation of Related Structures:
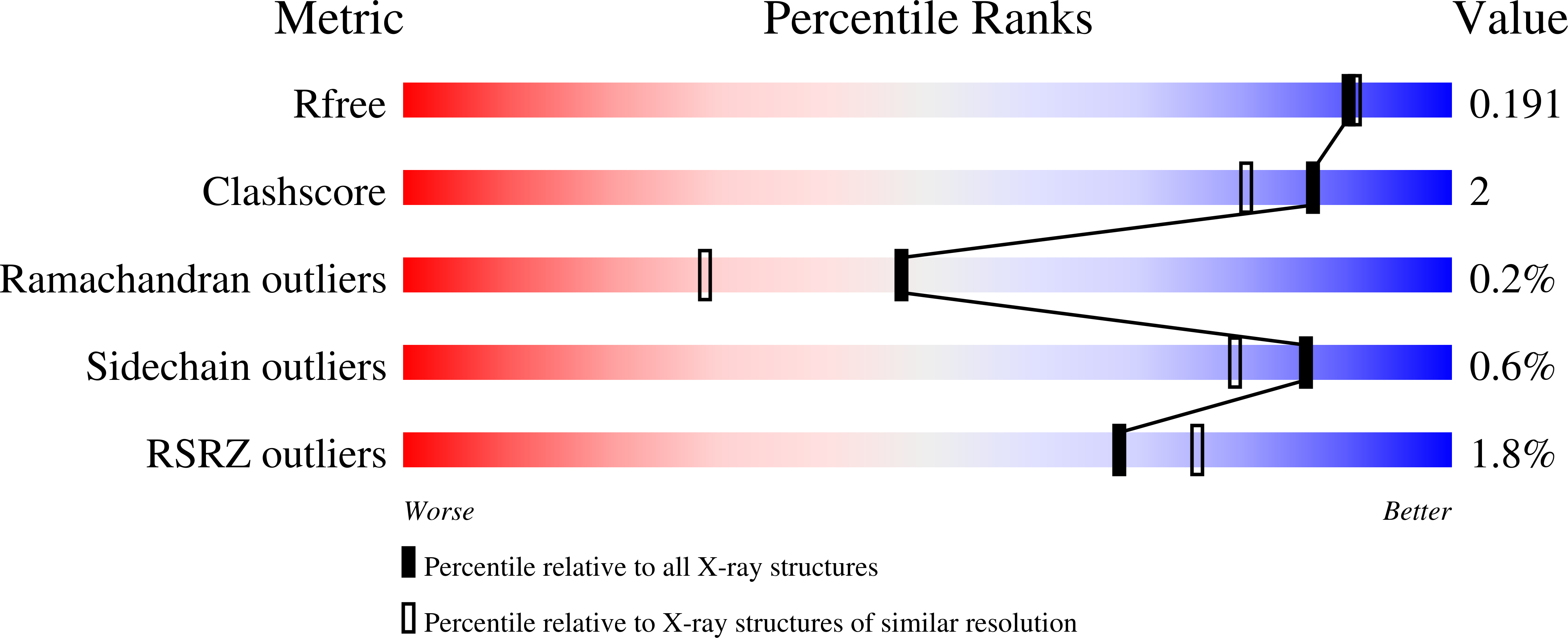
4EAM, 4EAN - PubMed Abstract:
Ligand-dependent activity has been engineered into enzymes for purposes ranging from controlling cell morphology to reprogramming cellular signaling pathways. Where these successes have typically fused a naturally allosteric domain to the enzyme of interest, here we instead demonstrate an approach for designing a de novo allosteric effector site directly into the catalytic domain of an enzyme. This approach is distinct from traditional chemical rescue of enzymes in that it relies on disruption and restoration of structure, rather than active site chemistry, as a means to achieve modulate function. We present two examples, W33G in a β-glycosidase enzyme (β-gly) and W492G in a β-glucuronidase enzyme (β-gluc), in which we engineer indole-dependent activity into enzymes by removing a buried tryptophan side chain that serves as a buttress for the active site architecture. In both cases, we observe a loss of function, and in both cases we find that the subsequent addition of indole can be used to restore activity. Through a detailed analysis of β-gly W33G kinetics, we demonstrate that this rescued enzyme is fully functionally equivalent to the corresponding wild-type enzyme. We then present the apo and indole-bound crystal structures of β-gly W33G, which together establish the structural basis for enzyme inactivation and rescue. Finally, we use this designed switch to modulate β-glycosidase activity in living cells using indole. Disruption and recovery of protein structure may represent a general technique for introducing allosteric control into enzymes, and thus may serve as a starting point for building a variety of bioswitches and sensors.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, 1200 Sunnyside Avenue, Lawrence, Kansas 66045-7534, USA.