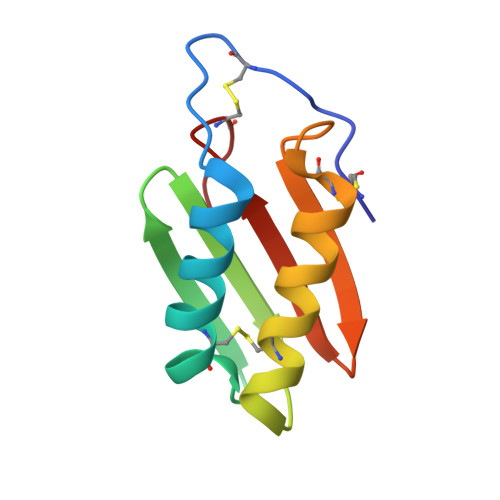
Atomic-resolution structure of the alpha-galactosyl binding Lyophyllum decastes lectin reveals a new protein family found in both fungi and plants.
van Eerde, A., Grahn, E.M., Winter, H.C., Goldstein, I.J., Krengel, U.(2015) Glycobiology 25: 492-501
- PubMed: 25504801
- DOI: https://doi.org/10.1093/glycob/cwu136
- Primary Citation of Related Structures:
4NDS, 4NDT, 4NDU, 4NDV - PubMed Abstract:
The crystal structure of the α-galactosyl binding Lyophyllum decastes lectin (LDL) was determined to 1.0 Å resolution by sulfur single-wavelength anomalous diffraction (SAD). The 10 kDa protein exhibits no sequence similarity to any protein with known structure and adopts a unique lectin fold, where a core of two antiparallel β-sheets at the heart of the homodimer is connected to the periphery of the structure by intramolecular disulfide bridges. This fold suggests that LDL is secreted, which sets it apart from other mushroom lectins. Structures of complexes between LDL and the ligands α-methylgalactoside and globotriose shed light on the binding specificity. Sequence comparison suggests a location and function of LDL and homologous proteins in or at the fungal cell wall. Structural comparison allows the identification of a superfamily of secreted proteins with the LDL fold, which may play a role at the interface between fungi and their environment.
Organizational Affiliation:
Department of Chemistry, University of Oslo, PO Box 1033, Oslo NO-0315, Norway.