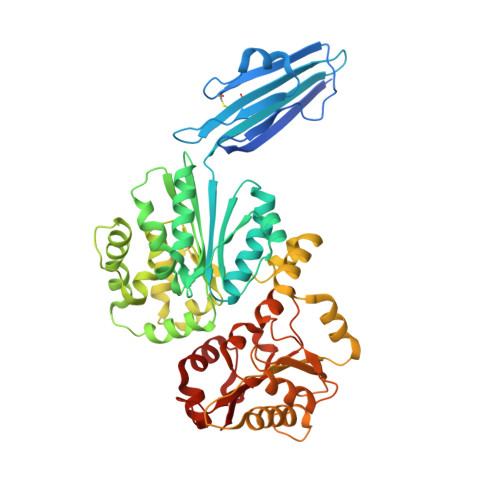
Crystal structures of sialyltransferase from Photobacterium damselae.
Huynh, N., Li, Y., Yu, H., Huang, S., Lau, K., Chen, X., Fisher, A.J.(2014) FEBS Lett 588: 4720-4729
- PubMed: 25451227
- DOI: https://doi.org/10.1016/j.febslet.2014.11.003
- Primary Citation of Related Structures:
4R83, 4R84, 4R9V - PubMed Abstract:
Sialyltransferase structures fall into either GT-A or GT-B glycosyltransferase fold. Some sialyltransferases from the Photobacterium genus have been shown to contain an additional N-terminal immunoglobulin (Ig)-like domain. Photobacterium damselae α2-6-sialyltransferase has been used efficiently in enzymatic and chemoenzymatic synthesis of α2-6-linked sialosides. Here we report three crystal structures of this enzyme. Two structures with and without a donor substrate analog CMP-3F(a)Neu5Ac contain an immunoglobulin (Ig)-like domain and adopt the GT-B sialyltransferase fold. The binary structure reveals a non-productive pre-Michaelis complex, which are caused by crystal lattice contacts that prevent the large conformational changes. The third structure lacks the Ig-domain. Comparison of the three structures reveals small inherent flexibility between the two Rossmann-like domains of the GT-B fold.
Organizational Affiliation:
Cell Biology Graduate Program, University of California, One Shields Avenue, Davis, CA 95616, USA.