An unexpected reactivity of the P460 cofactor in hydroxylamine oxidoreductase.
Dietl, A., Maalcke, W., Barends, T.R.(2015) Acta Crystallogr D Biol Crystallogr 71: 1708-1713
- PubMed: 26249351
- DOI: https://doi.org/10.1107/S1399004715010706
- Primary Citation of Related Structures:
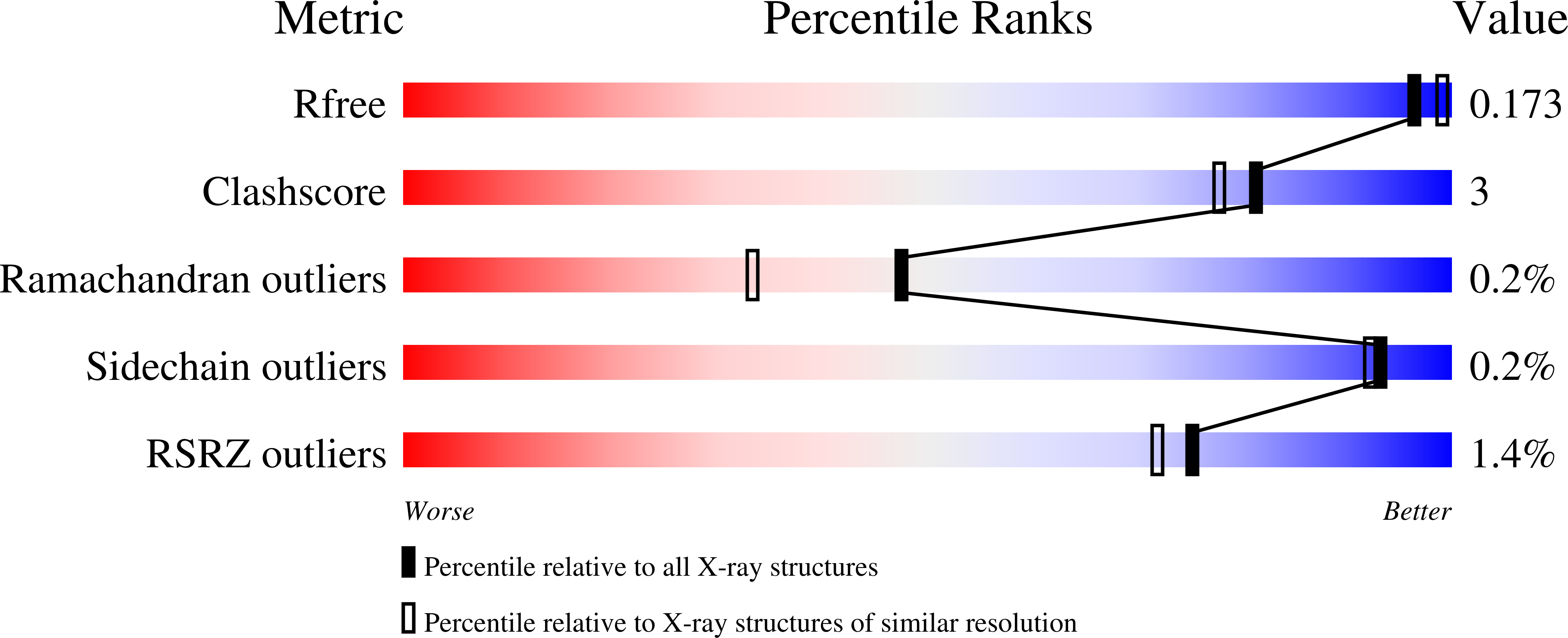
4RWM - PubMed Abstract:
Hydroxylamine oxidoreductases (HAOs) contain a unique haem cofactor called P460 that consists of a profoundly ruffled c-type haem with two covalent bonds between the haem porphyrin and a conserved tyrosine. This cofactor is exceptional in that it abstracts electrons from a ligand bound to the haem iron, whereas other haems involved in redox chemistry usually inject electrons into their ligands. The effects of the tyrosine cross-links and of the haem ruffling on the chemistry of this cofactor have been investigated theoretically but are not yet clear. A new crystal structure of an HAO from Candidatus Kuenenia stuttgartiensis, a model organism for anaerobic ammonium oxidation, now shows that its P460 cofactor has yet another unexpected reactivity: when ethylene glycol was used as a cryoprotectant, the 1.8 Å resolution electron-density maps showed additional density which could be interpreted as an ethylene glycol molecule covalently bound to the C16 atom of the haem ring, opposite the covalent links to the conserved tyrosine. Possible causes for this unexpected reactivity are discussed.
Organizational Affiliation:
Department of Biomolecular Mechanisms, Max Planck Institute for Medical Research, Jahnstrasse 29, 69120 Heidelberg, Germany.