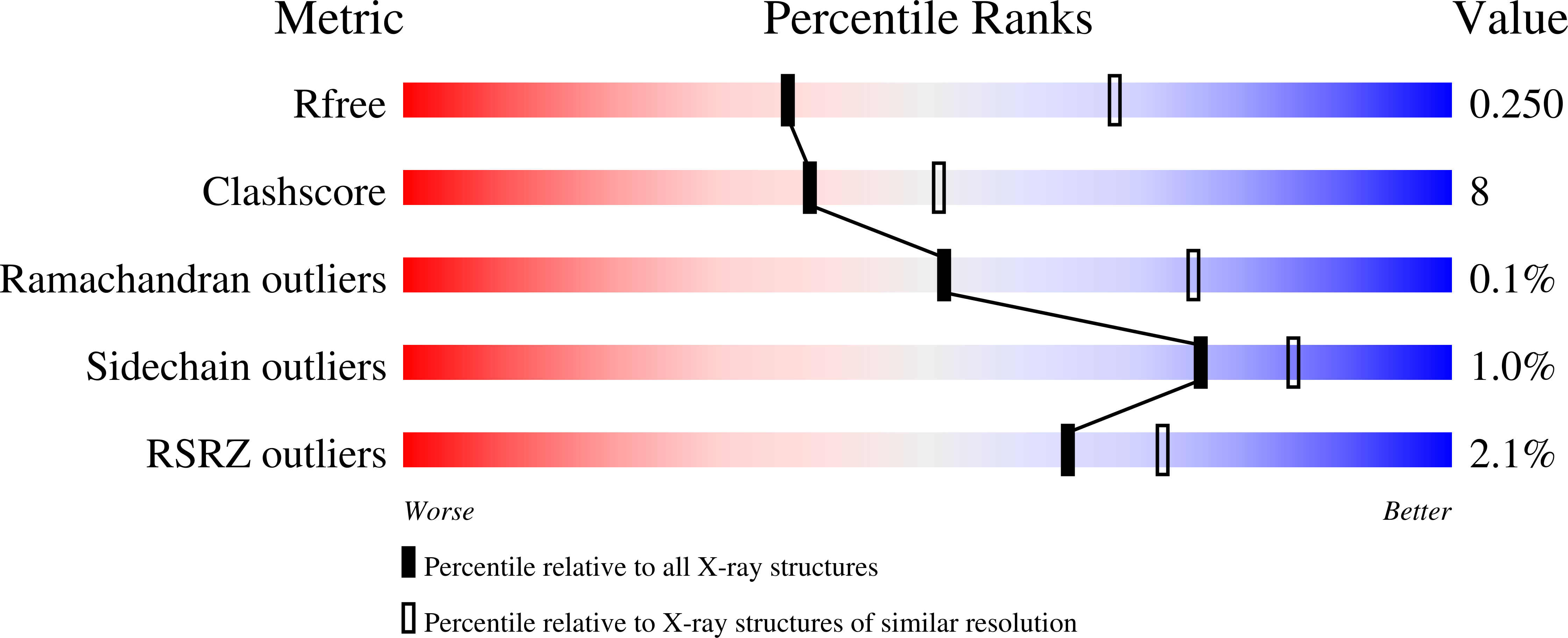
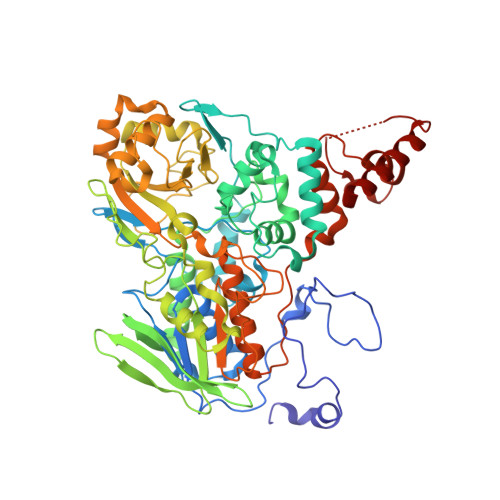
Structure-Based Mechanism of Oleate Hydratase from Elizabethkingia Meningoseptica.
Engleder, M., Pavkov-Keller, T., Emmerstorfer, A., Hromic, A., Schrempf, S., Steinkellner, G., Wriessnegger, T., Leitner, E., Strohmeier, G.A., Kaluzna, I., Mink, D., Schurmann, M., Wallner, S., Macheroux, P., Gruber, K., Pichler, H.(2015) Chembiochem 16: 1730
- PubMed: 26077980
- DOI: https://doi.org/10.1002/cbic.201500269
- Primary Citation of Related Structures:
4UIR - PubMed Abstract:
Hydratases provide access to secondary and tertiary alcohols by regio- and/or stereospecifically adding water to carbon-carbon double bonds. Thereby, hydroxy groups are introduced without the need for costly cofactor recycling, and that makes this approach highly interesting on an industrial scale. Here we present the first crystal structure of a recombinant oleate hydratase originating from Elizabethkingia meningoseptica in the presence of flavin adenine dinucleotide (FAD). A structure-based mutagenesis study targeting active site residues identified E122 and Y241 as crucial for the activation of a water molecule and for protonation of the double bond, respectively. Moreover, we also observed that two-electron reduction of FAD results in a sevenfold increase in the substrate hydration rate. We propose the first reaction mechanism for this enzyme class that explains the requirement for the flavin cofactor and the involvement of conserved amino acid residues in this regio- and stereoselective hydration.
Organizational Affiliation:
Institute of Molecular Biotechnology, NAWI Graz, Graz University of Technology, Petersgasse 14/2, 8010 Graz (Austria).