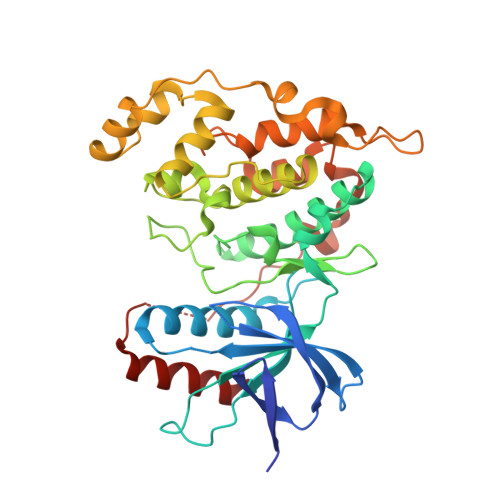
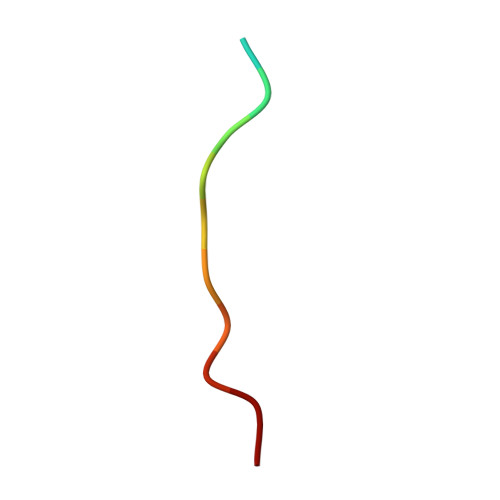
Structure and Dynamics of the Mkk7-Jnk Signaling Complex.
Kragelj, J., Palencia, A., Nanao, M.H., Maurin, D., Bouvignies, G., Blackledge, M., Jensen, M.R.(2015) Proc Natl Acad Sci U S A 112: 3409
- PubMed: 25737554
- DOI: https://doi.org/10.1073/pnas.1419528112
- Primary Citation of Related Structures:
4UX9 - PubMed Abstract:
Signaling specificity in the mitogen-activated protein kinase (MAPK) pathways is controlled by disordered domains of the MAPK kinases (MKKs) that specifically bind to their cognate MAPKs via linear docking motifs. MKK7 activates the c-Jun N-terminal kinase (JNK) pathway and is the only MKK containing three motifs within its regulatory domain. Here, we characterize the conformational behavior and interaction mechanism of the MKK7 regulatory domain. Using NMR spectroscopy, we develop an atomic resolution ensemble description of MKK7, revealing highly diverse intrinsic conformational propensities of the three docking sites, suggesting that prerecognition sampling of the bound-state conformation is not prerequisite for binding. Although the different sites exhibit similar affinities for JNK1, interaction kinetics differ considerably. Importantly, we determine the crystal structure of JNK1 in complex with the second docking site of MKK7, revealing two different binding modes of the docking motif correlating with observations from NMR exchange spectroscopy. Our results provide unique insight into how signaling specificity is regulated by linear motifs and, in general, into the role of conformational disorder in MAPK signaling.
Organizational Affiliation:
Université Grenoble Alpes, Centre National de la Recherche Scientifique, and Commissariat à l'Énergie Atomique et aux Énergies Alternatives, Institut de Biologie Structurale, F-38044 Grenoble, France; and.