The Interaction of RNA with Trap: The Role of Triplet Repeats and Separating Spacer Nucleotides
Hopcroft, N.H., Manfredo, A., Wendt, A.L., Brzozowski, A.M., Gollnick, P., Antson, A.A.(2004) J Mol Biol 338: 43
- PubMed: 15050822
- DOI: https://doi.org/10.1016/j.jmb.2004.02.038
- Primary Citation of Related Structures:
1UTD, 4V4F - PubMed Abstract:
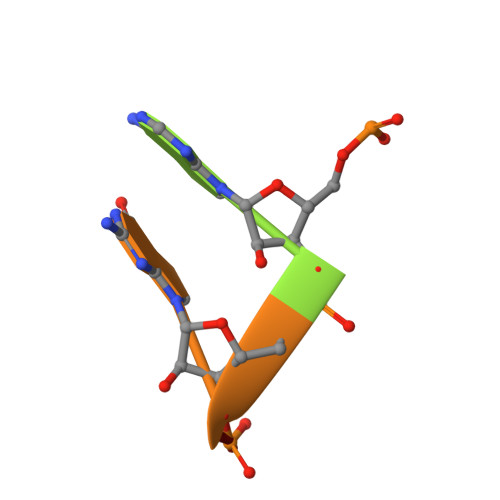
The trp RNA-binding attenuation protein (TRAP) regulates expression of the tryptophan biosynthetic genes in several Bacilli by binding to the leader region of the nascent trp mRNA, inhibiting continued transcription. The 11 subunit TRAP molecule is active in complex with tryptophan, and binds consequently an RNA target segment consisting of 11 (G/U)AG triplets, each separated by two or three non-conserved "spacer" nucleotides. Here, we report the first crystal structures of TRAP in a complex with RNA containing UAG triplets separated by two nucleotides and in a complex with RNA containing GAG triplets separated by three nucleotides. Comparison with known structures of TRAP-RNA complexes shows that both substitution of G-1 with U-1 in the triplet and addition of an extra spacer nucleotide lead to a more flexible complex. This suggests an explanation why, in the trp leader RNA, all three-nucleotide spacer regions are followed by a G-1 nucleotide. Taken together, the structures demonstrate that RNA binding to TRAP is mediated by specific interactions involving the A-2 and G-3 nucleotides of the triplet. This is accompanied by the disruption of stacking interactions between the bases of the other nucleotides, contributing to the increase in entropy that drives binding.
Organizational Affiliation:
York Structural Biology Laboratory, Department of Chemistry, University of York, Heslington, York YO10 5YW, UK.