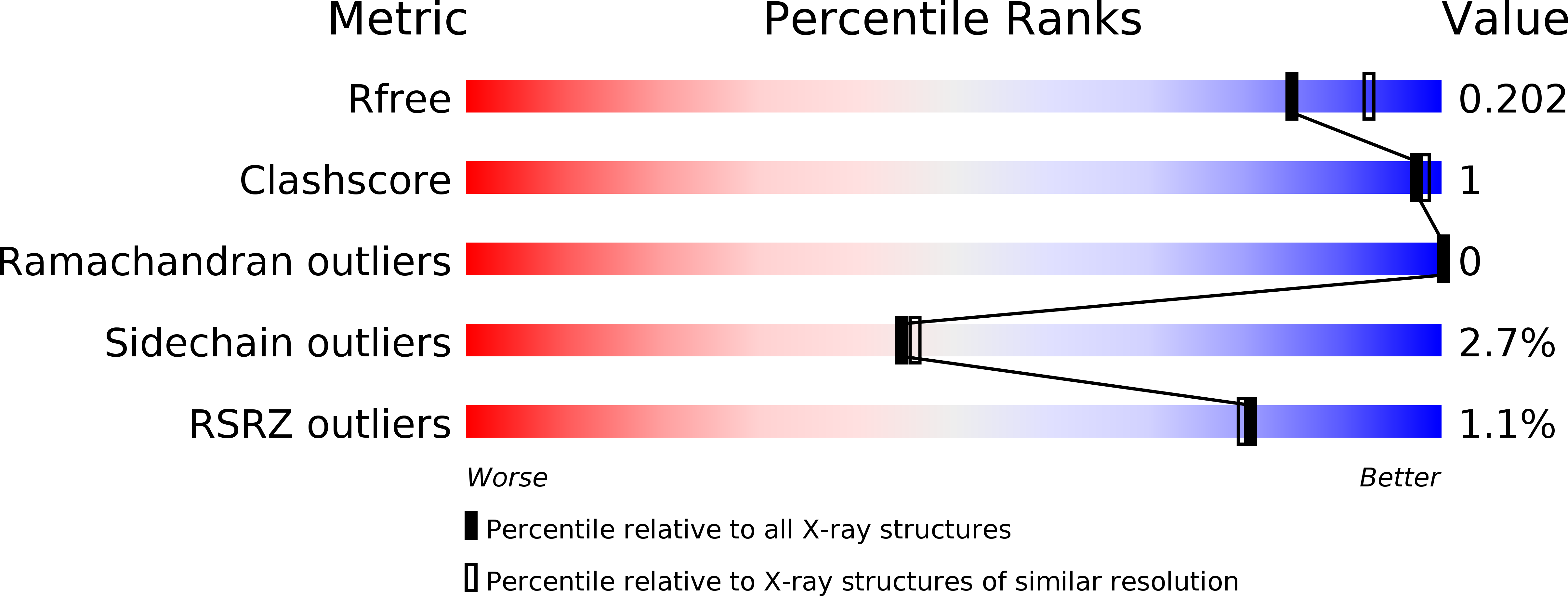
Natural flavonoids as antidiabetic agents. The binding of gallic and ellagic acids to glycogen phosphorylase b.
Kyriakis, E., Stravodimos, G.A., Kantsadi, A.L., Chatzileontiadou, D.S., Skamnaki, V.T., Leonidas, D.D.(2015) FEBS Lett 589: 1787-1794
- PubMed: 25980608
- DOI: https://doi.org/10.1016/j.febslet.2015.05.013
- Primary Citation of Related Structures:
4YUA, 4Z5X - PubMed Abstract:
We present a study on the binding of gallic acid and its dimer ellagic acid to glycogen phosphorylase (GP). Ellagic acid is a potent inhibitor with Kis of 13.4 and 7.5 μM, in contrast to gallic acid which displays Kis of 1.7 and 3.9 mM for GPb and GPa, respectively. Both compounds are competitive inhibitors with respect to the substrate, glucose-1-phoshate, and non-competitive to the allosteric activator, AMP. However, only ellagic acid functions with glucose in a strongly synergistic mode. The crystal structures of the GPb-gallic acid and GPb-ellagic acid complexes were determined at high resolution, revealing that both ligands bind to the inhibitor binding site of the enzyme and highlight the structural basis for the significant difference in their inhibitory potency.
Organizational Affiliation:
Department of Biochemistry and Biotechnology, University of Thessaly, 26 Ploutonos Str., 41221 Larissa, Greece.