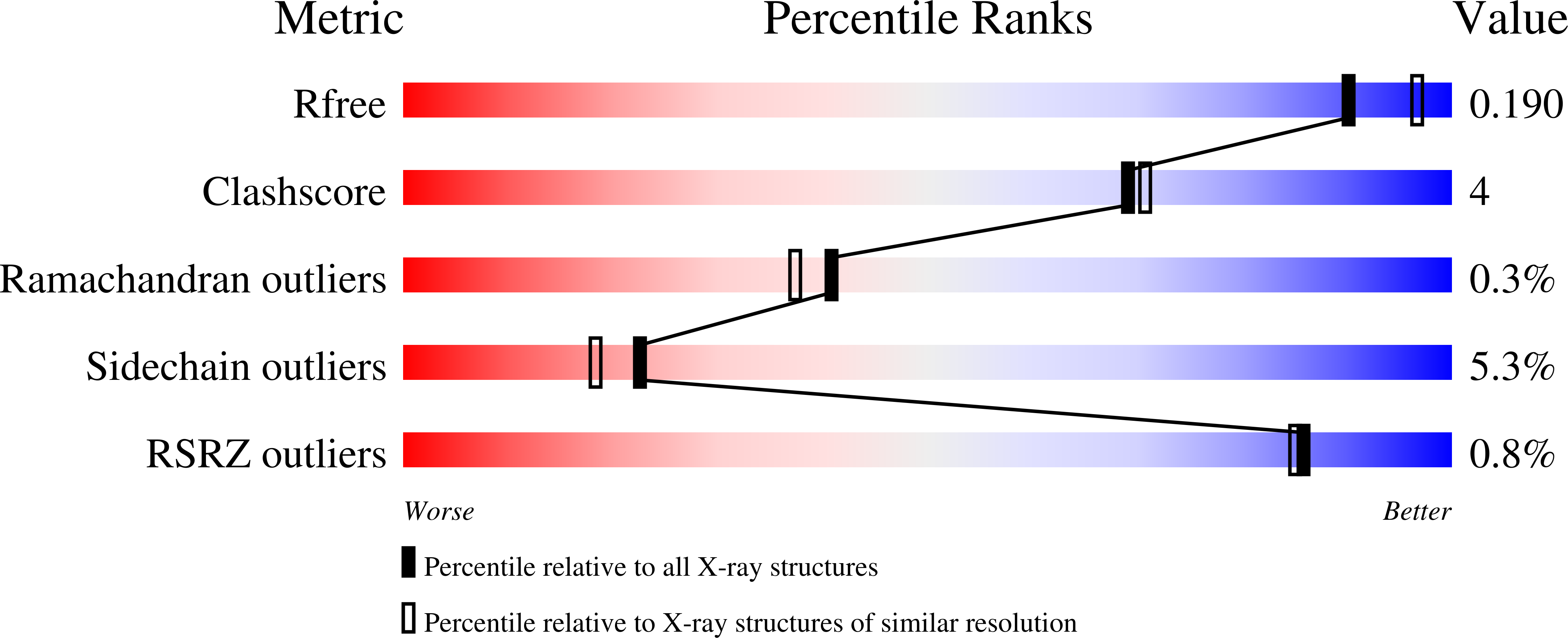
Inhibition of D-xylose isomerase by polyols: atomic details by joint X-ray/neutron crystallography.
Kovalevsky, A., Hanson, B.L., Mason, S.A., Forsyth, V.T., Fisher, Z., Mustyakimov, M., Blakeley, M.P., Keen, D.A., Langan, P.(2012) Acta Crystallogr D Biol Crystallogr 68: 1201-1206
- PubMed: 22948921
- DOI: https://doi.org/10.1107/S0907444912024808
- Primary Citation of Related Structures:
4DUO, 4DVO - PubMed Abstract:
D-Xylose isomerase (XI) converts the aldo-sugars xylose and glucose to their keto analogs xylulose and fructose, but is strongly inhibited by the polyols xylitol and sorbitol, especially at acidic pH. In order to understand the atomic details of polyol binding to the XI active site, a 2.0 Å resolution room-temperature joint X-ray/neutron structure of XI in complex with Ni(2+) cofactors and sorbitol inhibitor at pH 5.9 and a room-temperature X-ray structure of XI containing Mg(2+) ions and xylitol at the physiological pH of 7.7 were obtained. The protonation of oxygen O5 of the inhibitor, which was found to be deprotonated and negatively charged in previous structures of XI complexed with linear glucose and xylulose, was directly observed. The Ni(2+) ions occupying the catalytic metal site (M2) were found at two locations, while Mg(2+) in M2 is very mobile and has a high B factor. Under acidic conditions sorbitol gains a water-mediated interaction that connects its O1 hydroxyl to Asp257. This contact is not found in structures at basic pH. The new interaction that is formed may improve the binding of the inhibitor, providing an explanation for the increased affinity of the polyols for XI at low pH.
Organizational Affiliation:
Bioscience Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA. [email protected]