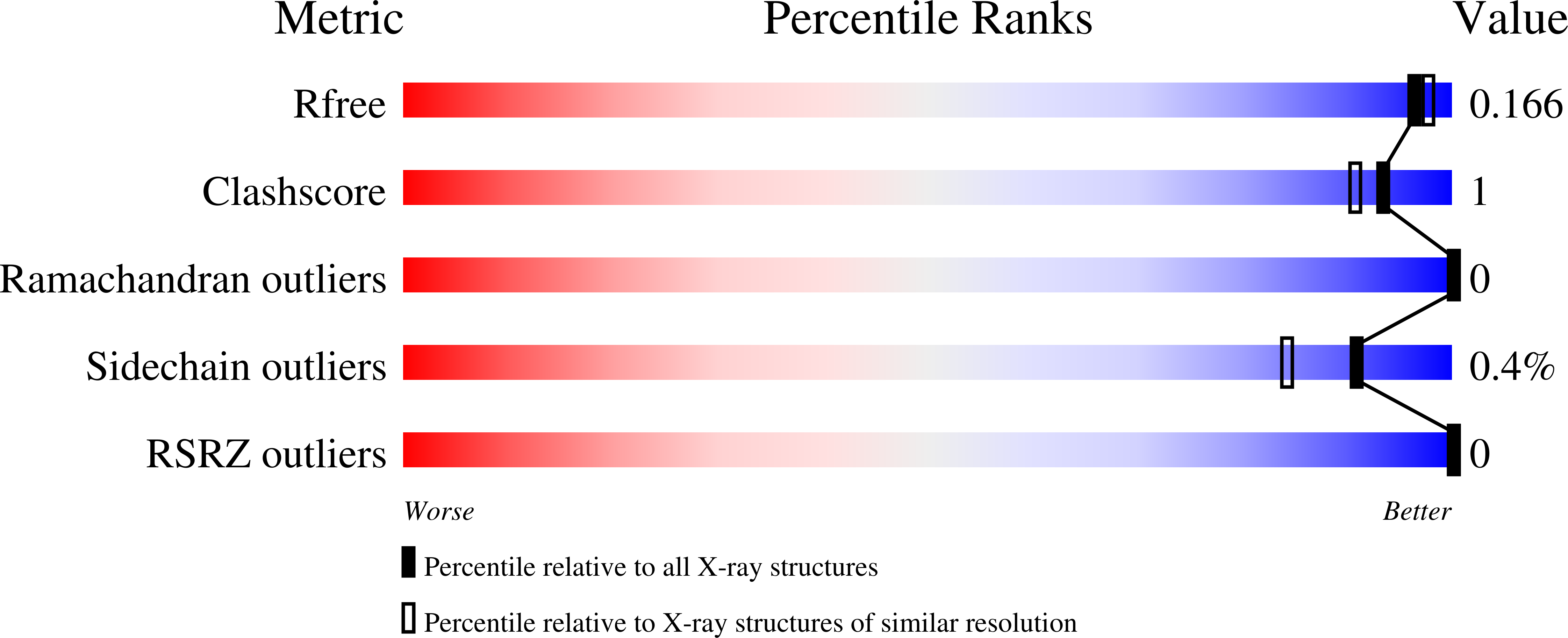
Contributions of a unique beta-clamp to substrate recognition illuminates the molecular basis of exolysis in ferulic acid esterases.
Gruninger, R.J., Cote, C., McAllister, T.A., Abbott, D.W.(2016) Biochem J 473: 839-849
- PubMed: 27026397
- DOI: https://doi.org/10.1042/BJ20151153
- Primary Citation of Related Structures:
5CXU, 5CXX - PubMed Abstract:
Lignocellulosic biomass is a promising renewable resource; however, deconstruction of this material is still the rate-limiting step. Major obstacles in the biocatalytic turnover of lignocellulose are ester-linked decorations that prevent access to primary structural polysaccharides. Enzymes targeting these esters represent promising biotools for increasing bioconversion efficiency. Ruminant livestock are unique in their ability to degrade lignocellulose through the action of their gut microbiome. The anaerobic fungi (phylum Neocallimastigomycota) are key members of this ecosystem that express a large repertoire of carbohydrate-active enzymes (CAZymes) with little sequence identity with characterized CAZymes [Lombard, Golaconda, Drula, Coutinho and Henrissat (2014) Nucleic Acids Res. 42: , D490-D495]. We have identified a carbohydrate esterase family 1 (CE1) ferulic acid esterase (FAE) belonging to Anaeromyces mucronatus(AmCE1/Fae1a), and determined its X-ray structure in both the presence [1.55 Å (1 Å=0.1 nm)] and absence (1.60 Å) of ferulic acid. AmCE1 adopts an α/β-hydrolase fold that is structurally conserved with bacterial FAEs, and possesses a unique loop, termed the β-clamp, that encloses the ligand. Isothermal titration calorimetry reveals that substrate binding is driven by enthalpic contributions, which overcomes a large entropic penalty. A comparative analysis of AmCE1 with related enzymes has uncovered the apparent structural basis for differential FAE activities targeting cross-linking ferulic acid conjugates compared with terminal decorations. Based on comparisons to structurally characterized FAEs, we propose that the β-clamp may define the structural basis of exolytic activities in FAEs. This provides a structure-based tool for predicting exolysis and endolysis in CE1. These insights hold promise for rationally identifying enzymes tailored for bioconversion of biomass with variations in cell wall composition.
Organizational Affiliation:
Lethbridge Research Centre, Agriculture and Agri-Food Canada, 5403-1st Ave South, Lethbridge, AB, Canada, T1J 4B1 [email protected] [email protected].