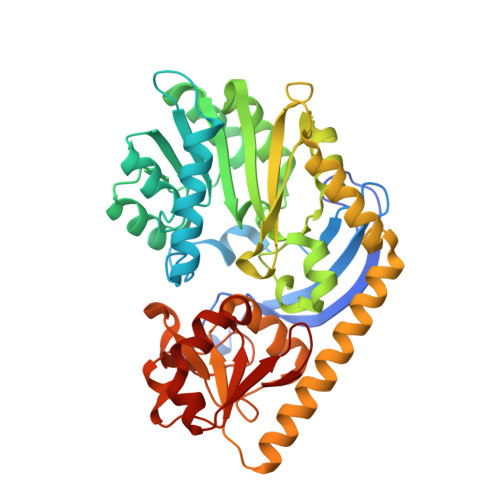
Structural studies on KijD1, a sugar C-3'-methyltransferase.
Dow, G.T., Thoden, J.B., Holden, H.M.(2016) Protein Sci 25: 2282-2289
- PubMed: 27595766
- DOI: https://doi.org/10.1002/pro.3034
- Primary Citation of Related Structures:
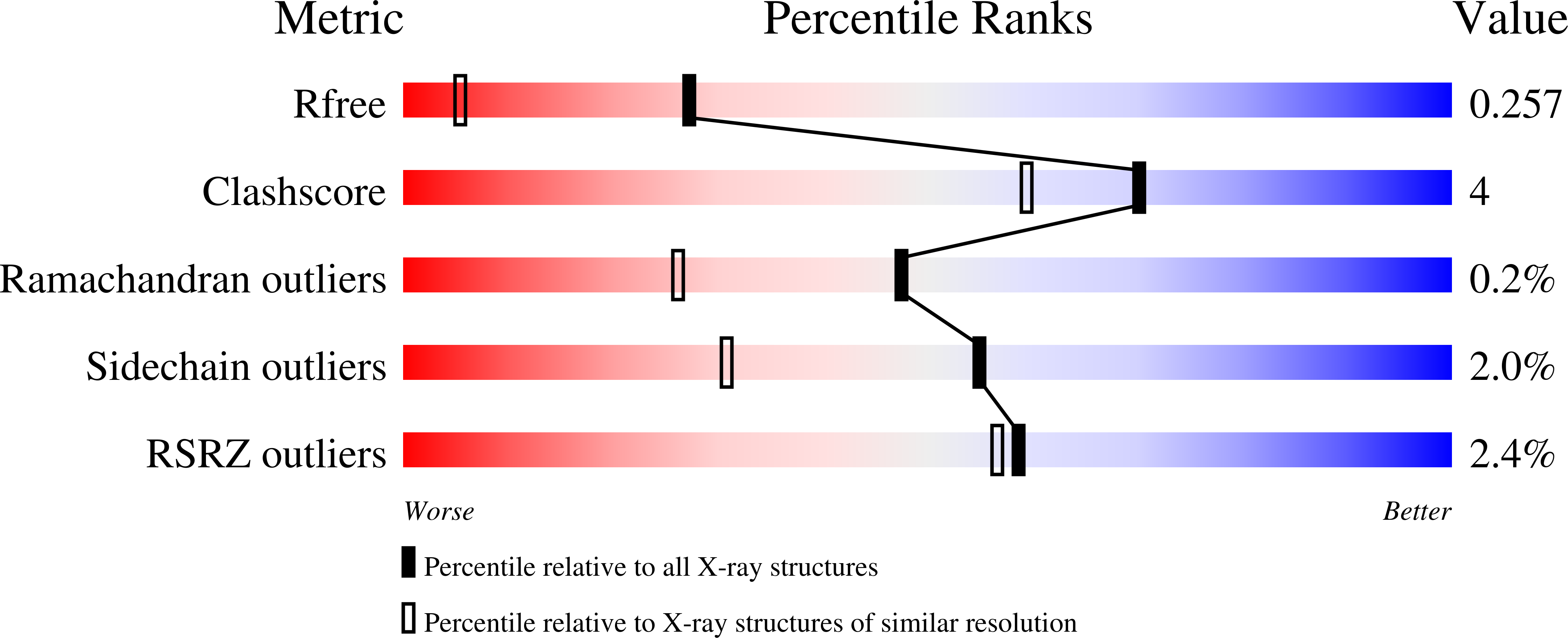
5T64, 5T67, 5T6B - PubMed Abstract:
Kijanimicin is an antitumor antibiotic isolated from Actinomadura kijaniata. It is composed of three distinct moieties: a pentacyclic core, a monosaccharide referred to as d-kijanose, and a tetrasaccharide chain composed of l-digitoxose units. d-Kijanose is a highly unusual nitro-containing tetradeoxysugar, which requires at least ten enzymes for its production. Here we describe a structural analysis of one of these enzymes, namely KijD1, which functions as a C-3'-methyltransferase using S-adenosylmethionine as its cofactor. For this investigation, two ternary complexes of KijD1, determined in the presence of S-adenosylhomocysteine (SAH) and dTDP or SAH and dTDP-3-amino-2,3,6-trideoxy-4-keto-3-methyl-d-glucose, were solved to 1.7 or 1.6 Å resolution, respectively. Unexpectedly, these structures, as well as additional biochemical analyses, demonstrated that the quaternary structure of KijD1 is a dimer. Indeed, this is in sharp contrast to that previously observed for the sugar C-3'-methyltransferase isolated from Micromonospora chalcea. By the judicious use of site-directed mutagenesis, it was possible to convert the dimeric form of KijD1 into a monomeric version. The quaternary structure of KijD1 could not have been deduced based solely on bioinformatics approaches, and thus this investigation highlights the continuing need for experimental validation.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin, Madison, WI, 53706.