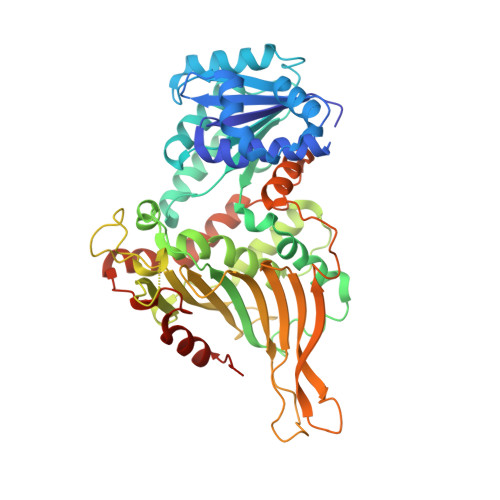
Mutations in the tetramer interface of human glucose-6-phosphate dehydrogenase reveals kinetic differences between oligomeric states.
Ranzani, A.T., Cordeiro, A.T.(2017) FEBS Lett 591: 1278-1284
- PubMed: 28370139
- DOI: https://doi.org/10.1002/1873-3468.12638
- Primary Citation of Related Structures:
5UKW - PubMed Abstract:
Glucose-6-phosphate dehydrogenase (G6PDH) catalyzes the oxidation of glucose-6-phoshate to 6-phospho-gluconolactone with the concomitant reduction of NADP + to NADPH. In solution, the recombinant human G6PDH is known to be active as dimers and tetramers. To distinguish between the kinetic properties of dimers and tetramers of the G6PDH is not trivial. Steady-state kinetic experiments are often performed at low enzyme concentrations, which favor the dimeric state. The present work describes two novel human G6PDH mutants, one that creates four disulfide bonds among apposing dimers, resulting in a 'cross-linked' tetramer, and another that prevents the dimer to dimer association. The functional and structural characterizations of such mutants indicate the tetramer as the most active form of human G6PDH.
Organizational Affiliation:
Institute of Biology, University of Campinas, Sao Paulo, Brazil.