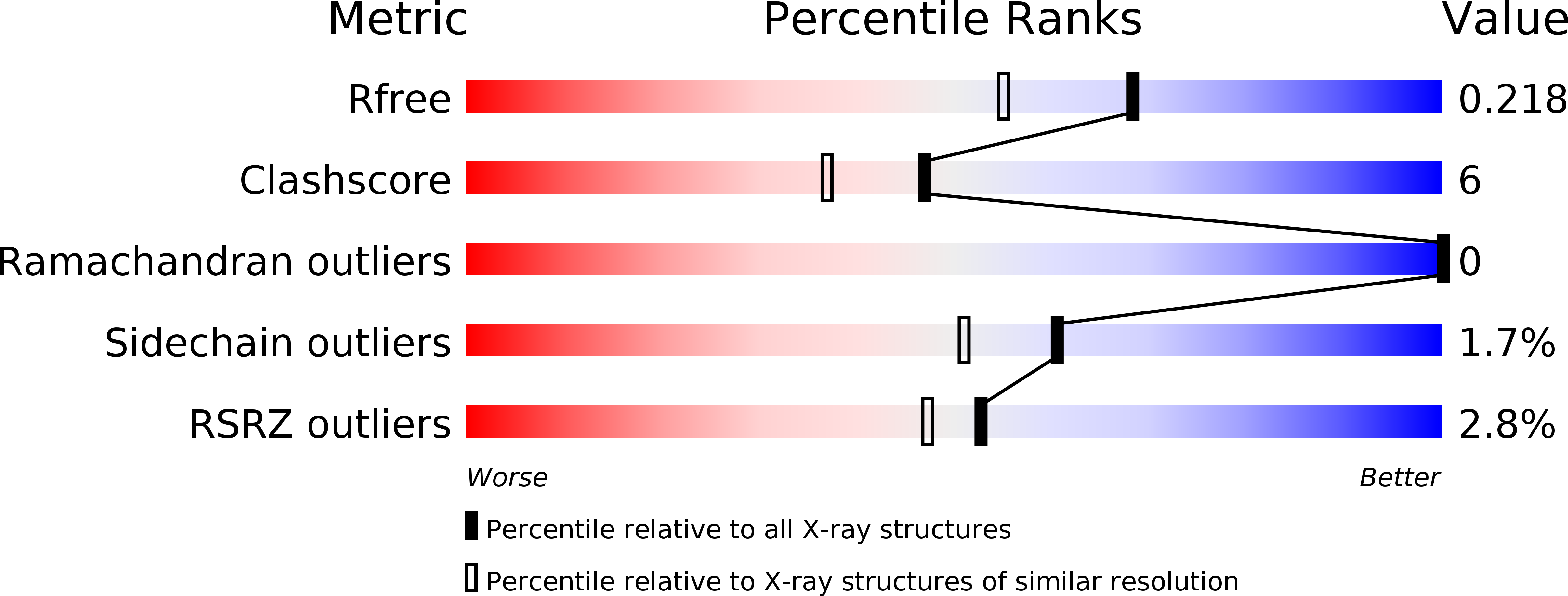
Molecular basis for the substrate specificity of quorum signal synthases.
Dong, S.H., Frane, N.D., Christensen, Q.H., Greenberg, E.P., Nagarajan, R., Nair, S.K.(2017) Proc Natl Acad Sci U S A 114: 9092-9097
- PubMed: 28784791
- DOI: https://doi.org/10.1073/pnas.1705400114
- Primary Citation of Related Structures:
5W8A, 5W8C, 5W8D, 5W8E, 5W8G - PubMed Abstract:
In several Proteobacteria , LuxI-type enzymes catalyze the biosynthesis of acyl-homoserine lactones (AHL) signals using S -adenosyl-l-methionine and either cellular acyl carrier protein (ACP)-coupled fatty acids or CoA-aryl/acyl moieties as progenitors. Little is known about the molecular mechanism of signal biosynthesis, the basis for substrate specificity, or the rationale for donor specificity for any LuxI member. Here, we present several cocrystal structures of BjaI, a CoA-dependent LuxI homolog that represent views of enzyme complexes that exist along the reaction coordinate of signal synthesis. Complementary biophysical, structure-function, and kinetic analysis define the features that facilitate the unusual acyl conjugation with S -adenosylmethionine (SAM). We also identify the determinant that establishes specificity for the acyl donor and identify residues that are critical for acyl/aryl specificity. These results highlight how a prevalent scaffold has evolved to catalyze quorum signal synthesis and provide a framework for the design of small-molecule antagonists of quorum signaling.
Organizational Affiliation:
Department of Biochemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801.