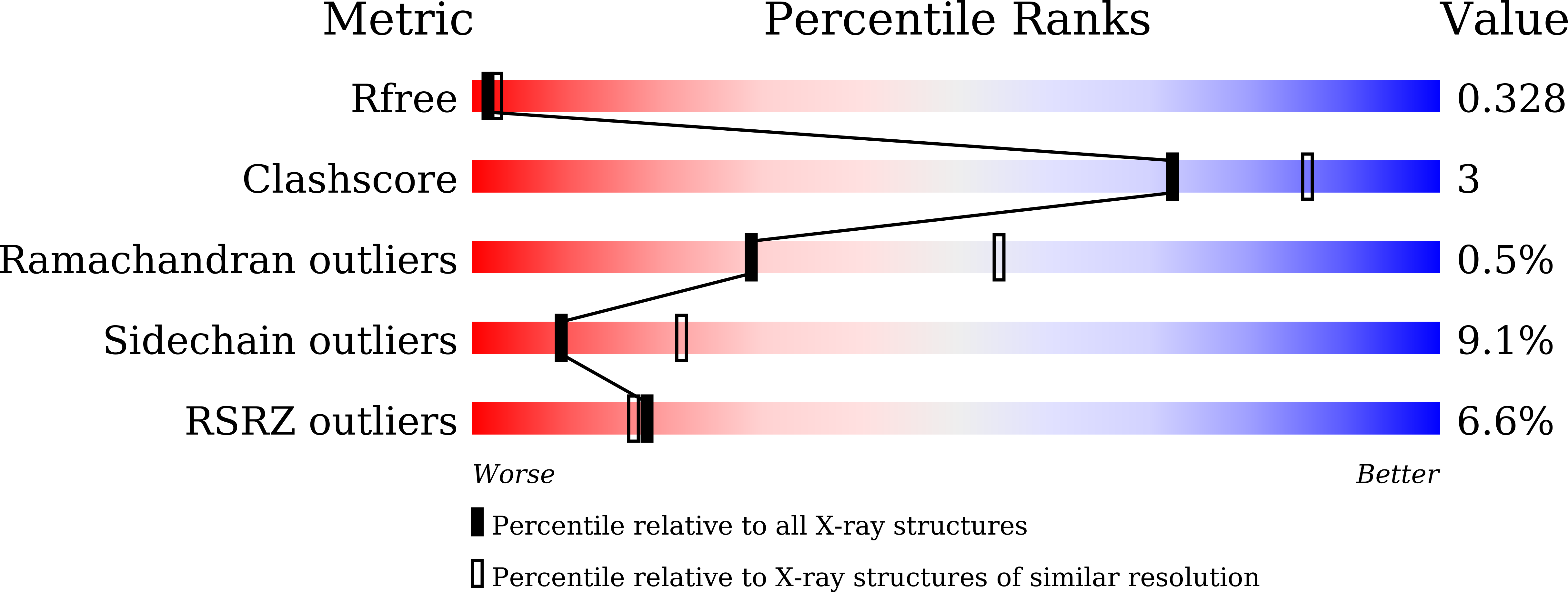
Direct observation of conformational population shifts in crystalline human hemoglobin.
Shibayama, N., Ohki, M., Tame, J.R.H., Park, S.Y.(2017) J Biol Chem 292: 18258-18269
- PubMed: 28931607
- DOI: https://doi.org/10.1074/jbc.M117.781146
- Primary Citation of Related Structures:
5X2R, 5X2S, 5X2T, 5X2U - PubMed Abstract:
Although X-ray crystallography is the most commonly used technique for studying the molecular structure of proteins, it is not generally able to monitor the dynamic changes or global domain motions that often underlie allostery. These motions often prevent crystal growth or reduce crystal order. We have recently discovered a crystal form of human hemoglobin that contains three protein molecules allowed to express a full range of quaternary structures, whereas maintaining strong X-ray diffraction. Here we use this crystal form to investigate the effects of two allosteric effectors, phosphate and bezafibrate, by tracking the structures and functions of the three hemoglobin molecules following the addition of each effector. The X-ray analysis shows that the addition of either phosphate or bezafibrate not only induces conformational changes in a direction from a relaxed-state to a tense-state, but also within relaxed-state populations. The microspectrophotometric O 2 equilibrium measurements on the crystals demonstrate that the binding of each effector energetically stabilizes the lowest affinity conformer more strongly than the intermediate affinity one, thereby reducing the O 2 affinity of tense-state populations, and that the addition of bezafibrate causes an ∼5-fold decrease in the O 2 affinity of relaxed-state populations. These results show that the allosteric pathway of hemoglobin involves shifts of populations rather than a unidirectional conversion of one quaternary structure to another, and that minor conformers of hemoglobin may have a disproportionate effect on the overall O 2 affinity.
Organizational Affiliation:
From the Department of Physiology, Division of Biophysics, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, Tochigi 329-0498 and [email protected].