Identification of a pyrophosphate-dependent kinase and its donor selectivity determinants.
Nagata, R., Fujihashi, M., Sato, T., Atomi, H., Miki, K.(2018) Nat Commun 9: 1765-1765
- PubMed: 29720581
- DOI: https://doi.org/10.1038/s41467-018-04201-z
- Primary Citation of Related Structures:
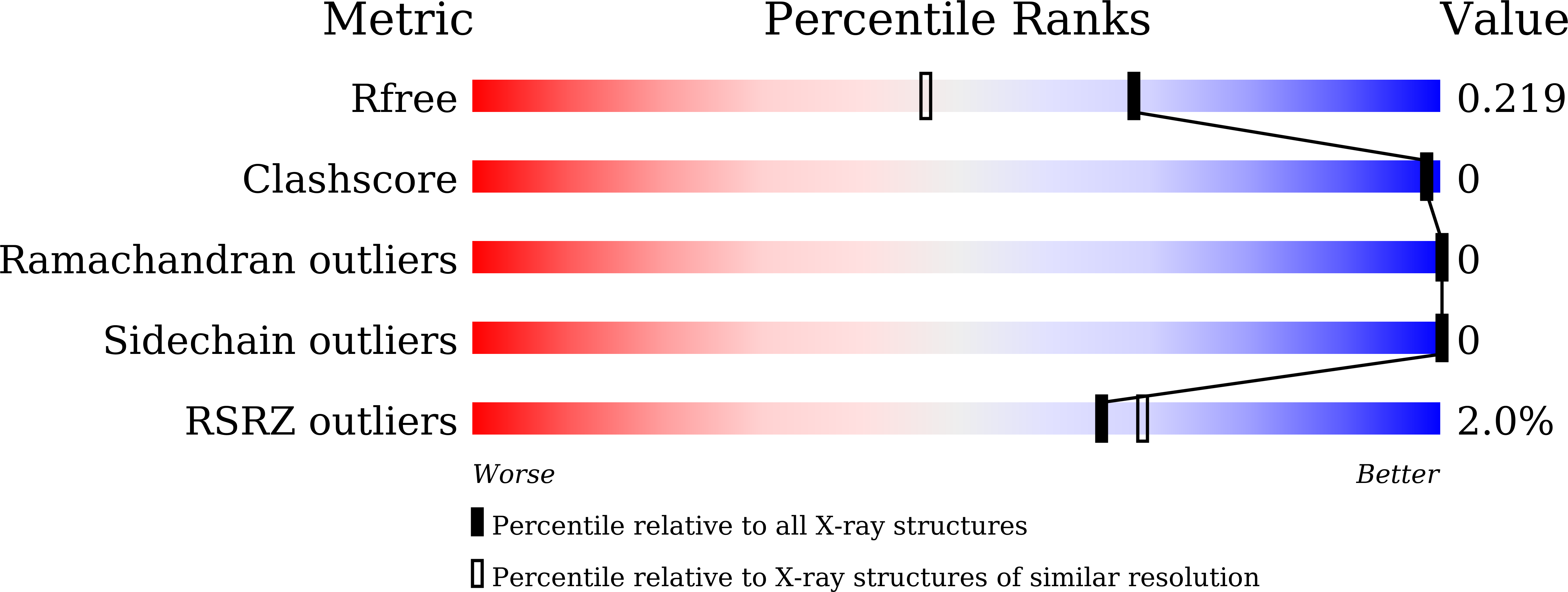
5YSP, 5YSQ - PubMed Abstract:
Almost all kinases utilize ATP as their phosphate donor, while a few kinases utilize pyrophosphate (PPi) instead. PPi-dependent kinases are often homologous to their ATP-dependent counterparts, but determinants of their different donor specificities remain unclear. We identify a PPi-dependent member of the ribokinase family, which differs from known PPi-dependent kinases, and elucidate its PPi-binding mode based on the crystal structures. Structural comparison and sequence alignment reveal five important residues: three basic residues specifically recognizing PPi and two large hydrophobic residues occluding a part of the ATP-binding pocket. Two of the three basic residues adapt a conserved motif of the ribokinase family for the PPi binding. Using these five key residues as a signature pattern, we discover additional PPi-specific members of the ribokinase family, and thus conclude that these residues are the determinants of PPi-specific binding. Introduction of these residues may enable transformation of ATP-dependent ribokinase family members into PPi-dependent enzymes.
Organizational Affiliation:
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502, Japan.