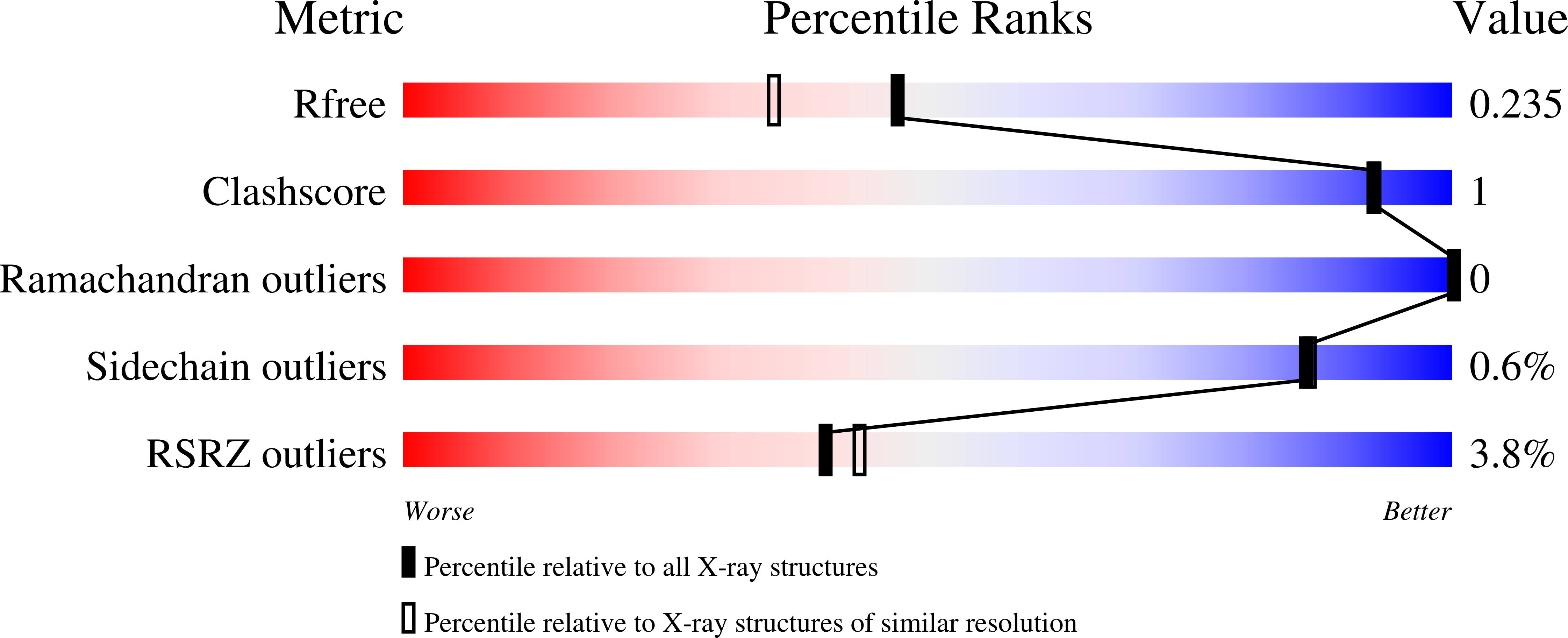
Crystal structure of thermospermine synthase fromMedicago truncatulaand substrate discriminatory features of plant aminopropyltransferases.
Sekula, B., Dauter, Z.(2018) Biochem J 475: 787-802
- PubMed: 29367265
- DOI: https://doi.org/10.1042/BCJ20170900
- Primary Citation of Related Structures:
6BQ2, 6BQ3, 6BQ4, 6BQ5, 6BQ6, 6BQ7 - PubMed Abstract:
Polyamines are linear polycationic compounds that play a crucial role in the growth and development of higher plants. One triamine (spermidine, SPD) and two tetraamine isomers (spermine, SPM, and thermospermine, TSPM) are obtained by the transfer of the aminopropyl group from decarboxylated S -adenosylmethionine to putrescine and SPD. These reactions are catalyzed by the specialized aminopropyltransferases. In that respect, plants are unique eukaryotes that have independently evolved two enzymes, thermospermine synthase (TSPS), encoded by the gene ACAULIS5 , and spermine synthase, which produce TSPM and SPM, respectively. In this work, we structurally characterize the ACAULIS5 gene product, TSPS, from the model legume plant Medicago truncatula ( Mt ). Six crystal structures of Mt TSPS - one without ligands and five in complexes with either reaction substrate (SPD), reaction product (TSPM), or one of three cofactor analogs (5'-methylthioadenosine, S -adenosylthiopropylamine, and adenosine) - give detailed insights into the biosynthesis of TSPM. Combined with small-angle X-ray scattering data, the crystal structures show that Mt TSPS is a symmetric homotetramer with an interdomain eight-stranded β-barrel. Such an assembly and the presence of a hinge-like feature between N-terminal and C-terminal domains give the protein additional flexibility which potentially improves loading substrates and discarding products after the catalytic event. We also discuss the sequence and structural features around the active site of the plant aminopropyltransferases that distinguish them from each other and determine their characteristic substrate discrimination.
Organizational Affiliation:
Synchrotron Radiation Research Section of Macromolecular Crystallography Laboratory, National Cancer Institute, South Cass Avenue 9700, Argonne, IL 60439, U.S.A. [email protected].