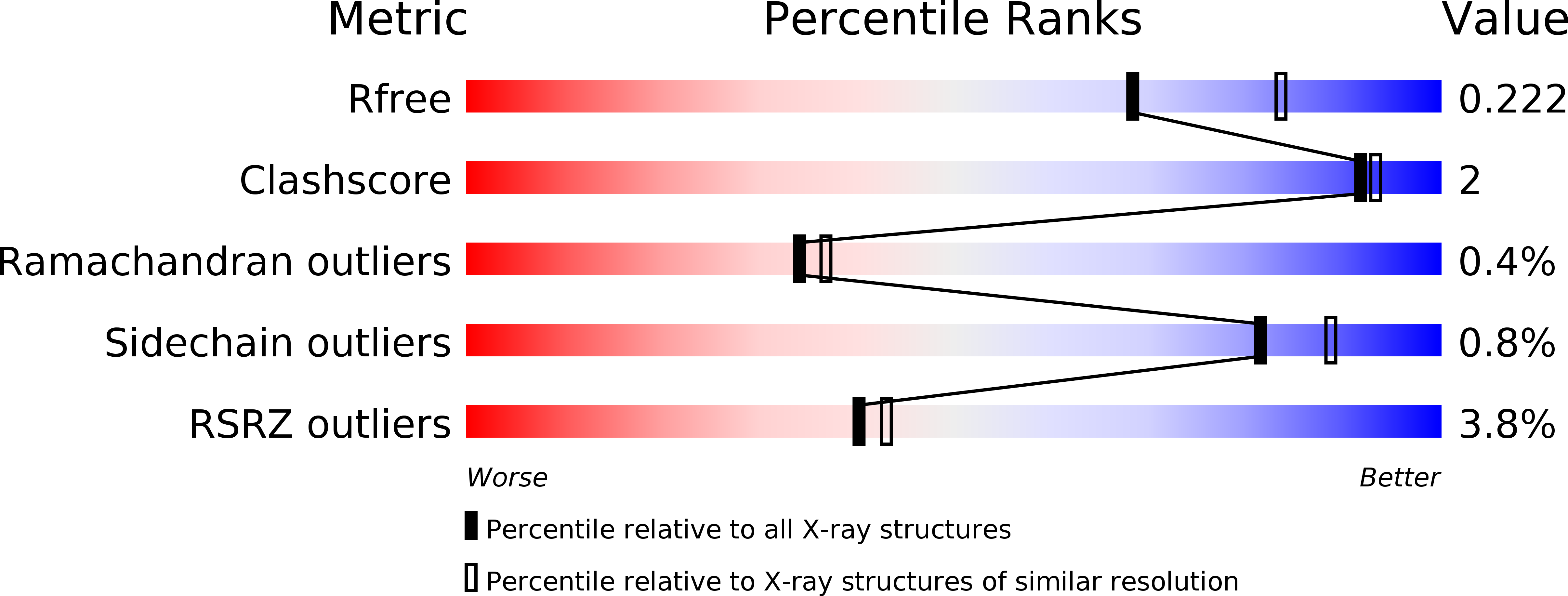
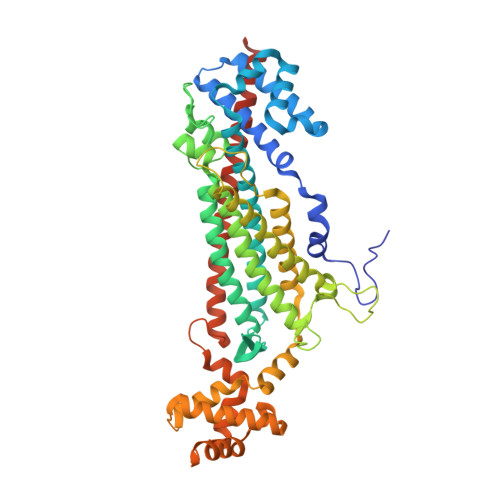
Structural Basis for the Catalytic Mechanism of Ethylenediamine- N, N'-disuccinic Acid Lyase, a Carbon-Nitrogen Bond-Forming Enzyme with a Broad Substrate Scope.
Poddar, H., de Villiers, J., Zhang, J., Puthan Veetil, V., Raj, H., Thunnissen, A.W.H., Poelarends, G.J.(2018) Biochemistry 57: 3752-3763
- PubMed: 29741885
- DOI: https://doi.org/10.1021/acs.biochem.8b00406
- Primary Citation of Related Structures:
6G3D, 6G3E, 6G3F, 6G3G, 6G3H, 6G3I - PubMed Abstract:
The natural aminocarboxylic acid product ethylenediamine- N, N'-disuccinic acid [( S, S)-EDDS] is able to form a stable complex with metal ions, making it an attractive biodegradable alternative for the synthetic metal chelator ethylenediaminetetraacetic acid (EDTA), which is currently used on a large scale in numerous applications. Previous studies have demonstrated that biodegradation of ( S, S)-EDDS may be initiated by an EDDS lyase, converting ( S, S)-EDDS via the intermediate N-(2-aminoethyl)aspartic acid (AEAA) into ethylenediamine and two molecules of fumarate. However, current knowledge of this enzyme is limited because of the absence of structural data. Here, we describe the identification and characterization of an EDDS lyase from Chelativorans sp. BNC1, which has a broad substrate scope, accepting various mono- and diamines for addition to fumarate. We report crystal structures of the enzyme in an unliganded state and in complex with formate, succinate, fumarate, AEAA, and ( S, S)-EDDS. The structures reveal a tertiary and quaternary fold that is characteristic of the aspartase/fumarase superfamily and support a mechanism that involves general base-catalyzed, sequential two-step deamination of ( S, S)-EDDS. This work broadens our understanding of mechanistic diversity within the aspartase/fumarase superfamily and will aid in the optimization of EDDS lyase for asymmetric synthesis of valuable (metal-chelating) aminocarboxylic acids.
Organizational Affiliation:
Department of Chemical and Pharmaceutical Biology, Groningen Research Institute of Pharmacy , University of Groningen , Antonius Deusinglaan 1 , 9713 AV Groningen , The Netherlands.