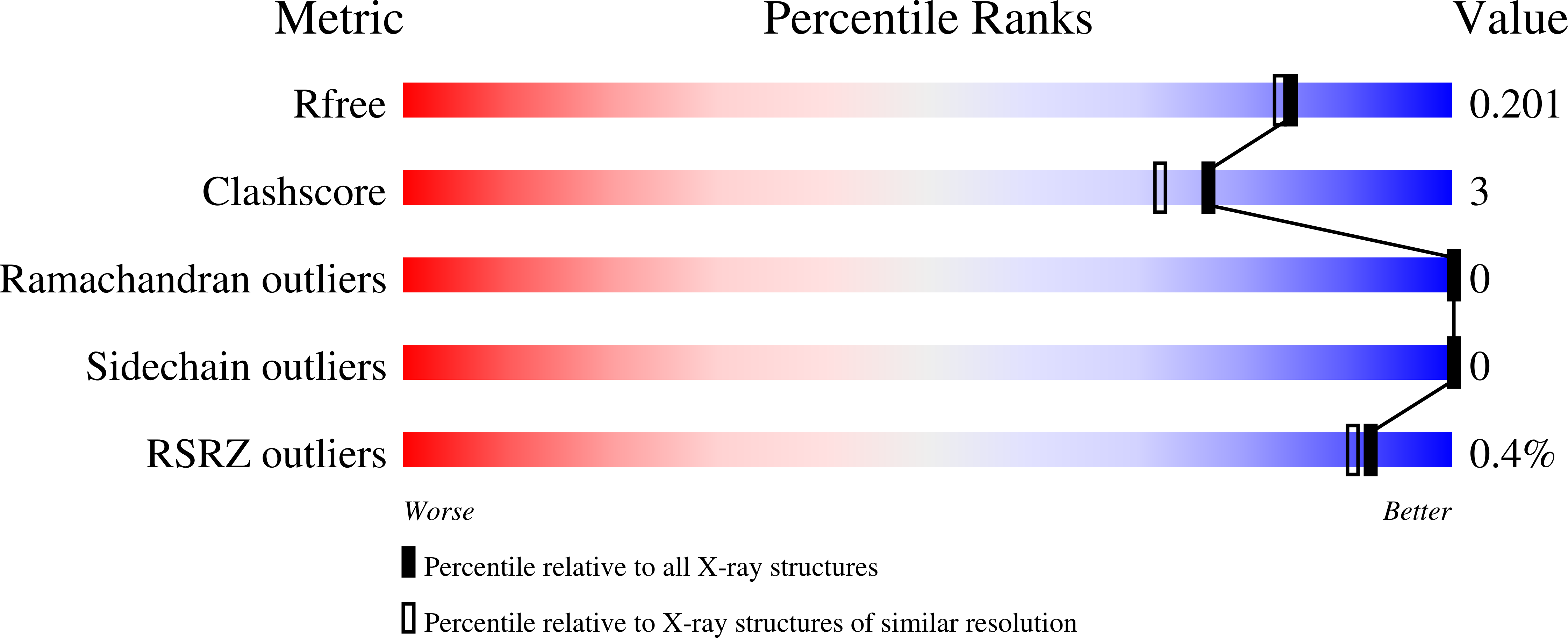
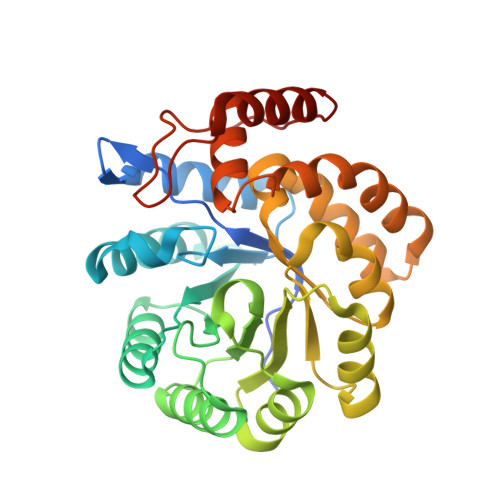
Asparagine-84, a regulatory allosteric site residue, helps maintain the quaternary structure of Campylobacter jejuni dihydrodipicolinate synthase.
Majdi Yazdi, M., Saran, S., Mrozowich, T., Lehnert, C., Patel, T.R., Sanders, D.A.R., Palmer, D.R.J.(2020) J Struct Biol 209: 107409-107409
- PubMed: 31678256
- DOI: https://doi.org/10.1016/j.jsb.2019.107409
- Primary Citation of Related Structures:
6TZU, 6U01 - PubMed Abstract:
Dihydrodipicolinate synthase (DHDPS) from Campylobacter jejuni is a natively homotetrameric enzyme that catalyzes the first unique reaction of (S)-lysine biosynthesis and is feedback-regulated by lysine through binding to an allosteric site. High-resolution structures of the DHDPS-lysine complex have revealed significant insights into the binding events. One key asparagine residue, N84, makes hydrogen bonds with both the carboxyl and the α-amino group of the bound lysine. We generated two mutants, N84A and N84D, to study the effects of these changes on the allosteric site properties. However, under normal assay conditions, N84A displayed notably lower catalytic activity, and N84D showed no activity. Here we show that these mutations disrupt the quaternary structure of DHDPS in a concentration-dependent fashion, as demonstrated by size-exclusion chromatography, multi-angle light scattering, dynamic light scattering, small-angle X-ray scattering (SAXS) and high-resolution protein crystallography.
Organizational Affiliation:
Department of Chemistry, University of Saskatchewan, 110 Science Place, Saskatoon, SK S7N 5C9, Canada.