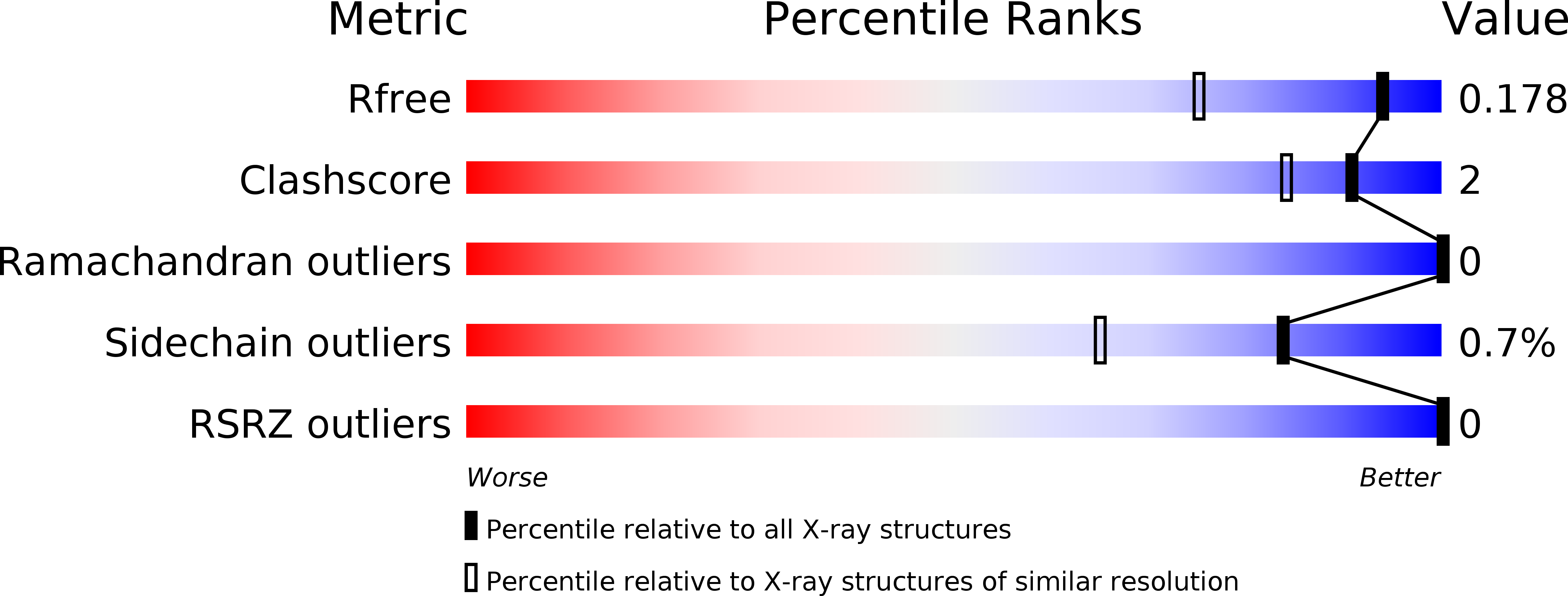
Bicyclic Boronates as Potent Inhibitors of AmpC, the Class C beta-Lactamase from Escherichia coli .
Lang, P.A., Parkova, A., Leissing, T.M., Calvopina, K., Cain, R., Krajnc, A., Panduwawala, T.D., Philippe, J., Fishwick, C.W.G., Trapencieris, P., Page, M.G.P., Schofield, C.J., Brem, J.(2020) Biomolecules 10
- PubMed: 32545682
- DOI: https://doi.org/10.3390/biom10060899
- Primary Citation of Related Structures:
6T3D, 6YEN, 6YEO, 6YPD - PubMed Abstract:
Resistance to β-lactam antibacterials, importantly via production of β-lactamases, threatens their widespread use. Bicyclic boronates show promise as clinically useful, dual-action inhibitors of both serine- (SBL) and metallo- (MBL) β-lactamases. In combination with cefepime, the bicyclic boronate taniborbactam is in phase 3 clinical trials for treatment of complicated urinary tract infections. We report kinetic and crystallographic studies on the inhibition of AmpC, the class C β‑lactamase from Escherichia coli , by bicyclic boronates, including taniborbactam, with different C-3 side chains. The combined studies reveal that an acylamino side chain is not essential for potent AmpC inhibition by active site binding bicyclic boronates. The tricyclic form of taniborbactam was observed bound to the surface of crystalline AmpC, but not at the active site, where the bicyclic form was observed. Structural comparisons reveal insights into why active site binding of a tricyclic form has been observed with the NDM-1 MBL, but not with other studied β-lactamases. Together with reported studies on the structural basis of inhibition of class A, B and D β‑lactamases, our data support the proposal that bicyclic boronates are broad-spectrum β‑lactamase inhibitors that work by mimicking a high energy 'tetrahedral' intermediate. These results suggest further SAR guided development could improve the breadth of clinically useful β-lactamase inhibition.
Organizational Affiliation:
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Oxford OX1 3TA, UK.