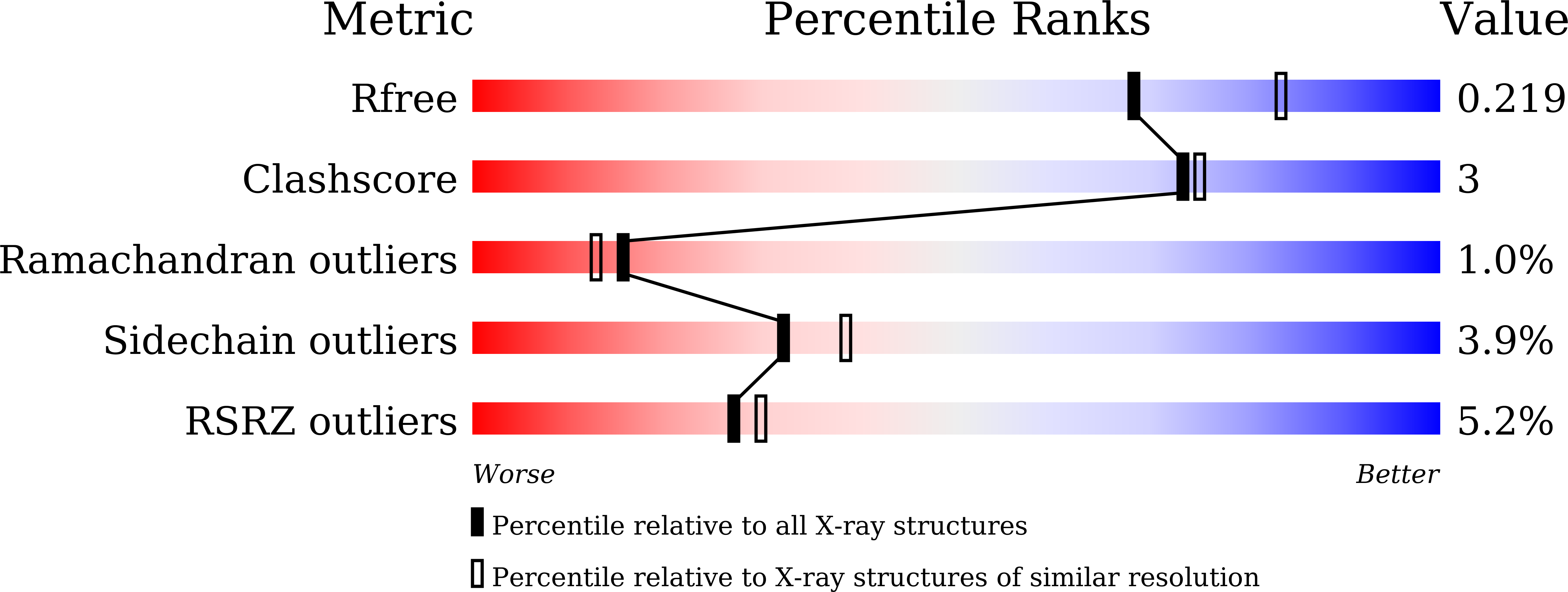
Structural studies reveal flexible roof of active site responsible for omega-transaminase CrmG overcoming by-product inhibition.
Xu, J., Tang, X., Zhu, Y., Yu, Z., Su, K., Zhang, Y., Dong, Y., Zhu, W., Zhang, C., Wu, R., Liu, J.(2020) Commun Biol 3: 455-455
- PubMed: 32814814
- DOI: https://doi.org/10.1038/s42003-020-01184-w
- Primary Citation of Related Structures:
6JC7, 6JC8, 6JC9, 6JCA, 6JCB - PubMed Abstract:
Amine compounds biosynthesis using ω-transaminases has received considerable attention in the pharmaceutical industry. However, the application of ω-transaminases was hampered by the fundamental challenge of severe by-product inhibition. Here, we report that ω-transaminase CrmG from Actinoalloteichus cyanogriseus WH1-2216-6 is insensitive to inhibition from by-product α-ketoglutarate or pyruvate. Combined with structural and QM/MM studies, we establish the detailed catalytic mechanism for CrmG. Our structural and biochemical studies reveal that the roof of the active site in PMP-bound CrmG is flexible, which will facilitate the PMP or by-product to dissociate from PMP-bound CrmG. Our results also show that amino acceptor caerulomycin M (CRM M), but not α-ketoglutarate or pyruvate, can form strong interactions with the roof of the active site in PMP-bound CrmG. Based on our results, we propose that the flexible roof of the active site in PMP-bound CrmG may facilitate CrmG to overcome inhibition from the by-product.
Organizational Affiliation:
State Key Laboratory of Respiratory Disease, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, 510530, Guangzhou, China. [email protected].