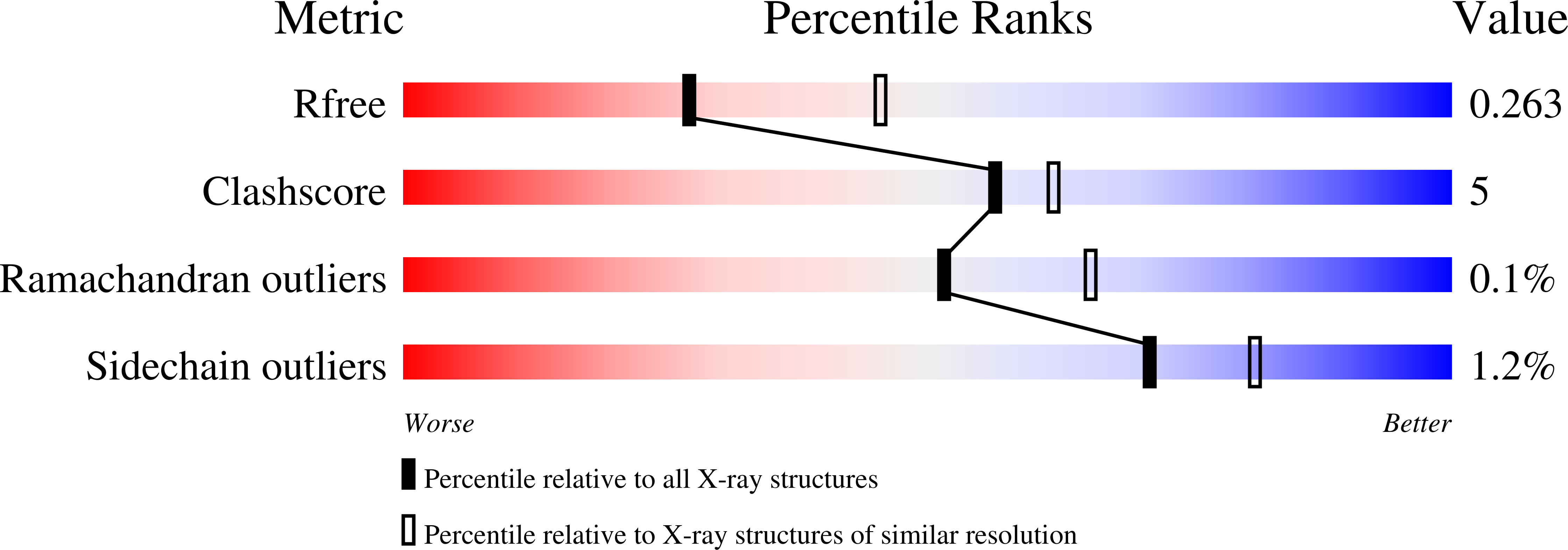
Discovery of a Potent Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase with Antioxidant Activity that Alleviates Alzheimer-like Pathology in Old APP/PS1 Mice.
Viayna, E., Coquelle, N., Cieslikiewicz-Bouet, M., Cisternas, P., Oliva, C.A., Sanchez-Lopez, E., Ettcheto, M., Bartolini, M., De Simone, A., Ricchini, M., Rendina, M., Pons, M., Firuzi, O., Perez, B., Saso, L., Andrisano, V., Nachon, F., Brazzolotto, X., Garcia, M.L., Camins, A., Silman, I., Jean, L., Inestrosa, N.C., Colletier, J.P., Renard, P.Y., Munoz-Torrero, D.(2021) J Med Chem 64: 812-839
- PubMed: 33356266
- DOI: https://doi.org/10.1021/acs.jmedchem.0c01775
- Primary Citation of Related Structures:
7AIS, 7AIT, 7AIU, 7AIV, 7AIW, 7AIX, 7AIY - PubMed Abstract:
The combination of the scaffolds of the cholinesterase inhibitor huprine Y and the antioxidant capsaicin results in compounds with nanomolar potencies toward human acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) that retain or improve the antioxidant properties of capsaicin. Crystal structures of their complexes with AChE and BChE revealed the molecular basis for their high potency. Brain penetration was confirmed by biodistribution studies in C57BL6 mice, with one compound ( 5i ) displaying better brain/plasma ratio than donepezil. Chronic treatment of 10 month-old APP/PS1 mice with 5i (2 mg/kg, i.p., 3 times per week, 4 weeks) rescued learning and memory impairments, as measured by three different behavioral tests, delayed the Alzheimer-like pathology progression, as suggested by a significantly reduced Aβ42/Aβ40 ratio in the hippocampus, improved basal synaptic efficacy, and significantly reduced hippocampal oxidative stress and neuroinflammation. Compound 5i emerges as an interesting anti-Alzheimer lead with beneficial effects on cognitive symptoms and on some underlying disease mechanisms.
Organizational Affiliation:
Laboratory of Medicinal Chemistry (CSIC Associated Unit), Faculty of Pharmacy and Food Sciences, and Institute of Biomedicine (IBUB), University of Barcelona, Av. Joan XXIII 27-31, E-08028 Barcelona, Spain.