Lard Injection Matrix for Serial Crystallography.
Nam, K.H.(2020) Int J Mol Sci 21
- PubMed: 32825186
- DOI: https://doi.org/10.3390/ijms21175977
- Primary Citation of Related Structures:
7CJZ, 7CK0 - PubMed Abstract:
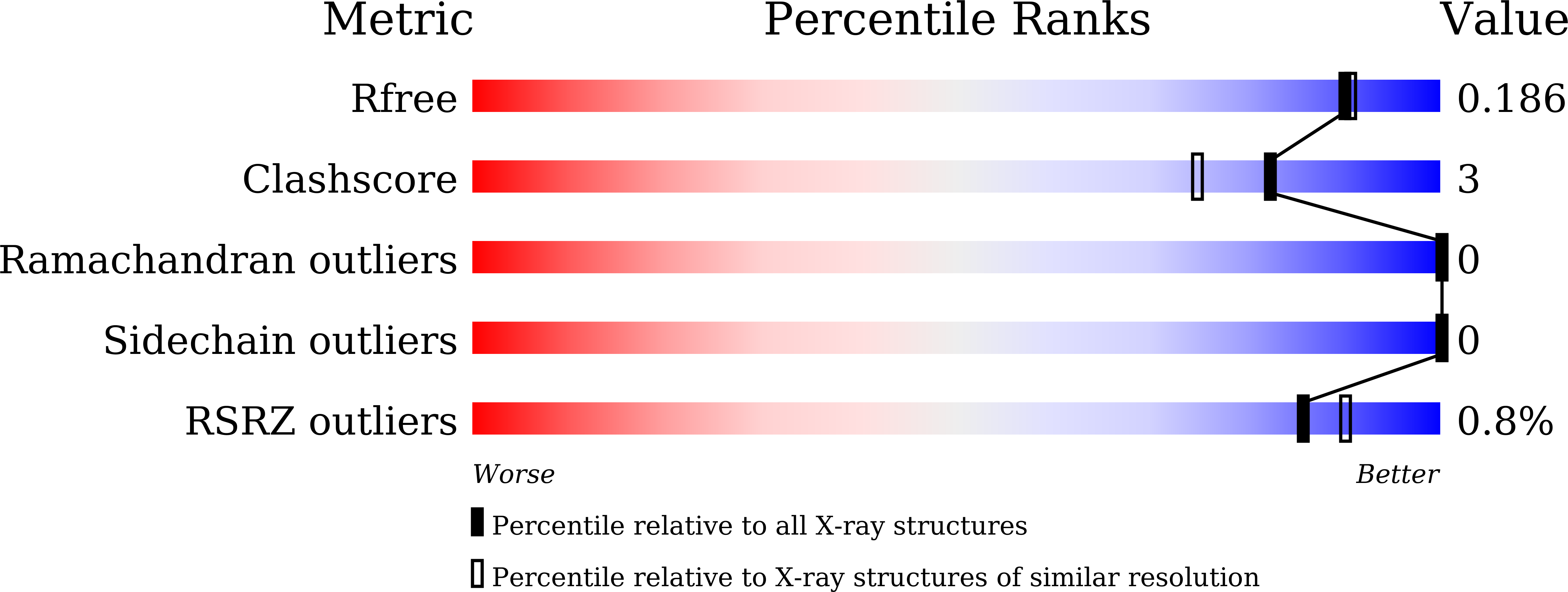
Serial crystallography (SX) using X-ray free electron laser or synchrotron X-ray allows for the determination of structures, at room temperature, with reduced radiation damage. Moreover, it allows for the study of structural dynamics of macromolecules using a time-resolved pump-probe, as well as mix-and-inject experiments. Delivering a crystal sample using a viscous medium decreases sample consumption by lowering the flow rate while being extruded from the injector or syringe as compared to a liquid jet injector. Since the environment of crystal samples varies, continuous development of the delivery medium is important for extended SX applications. Herein, I report the preparation and characterization of a lard-based sample delivery medium for SX. This material was obtained using heat treatment, and then the soluble impurities were removed through phase separation. The lard injection medium was highly stable and could be injected via a syringe needle extruded at room temperature with a flow rate < 200 nL/min. Serial millisecond crystallography experiments were performed using lard, and the room temperature structures of lysozyme and glucose isomerase embedded in lard at 1.75 and 1.80 Å, respectively, were determined. The lard medium showed X-ray background scattering similar or relatively lower than shortenings and lipidic cubic phase; therefore, it can be used as sample delivery medium in SX experiments.
Organizational Affiliation:
Department of Life Science, Pohang University of Science and Technology, Pohang 37673, Korea.