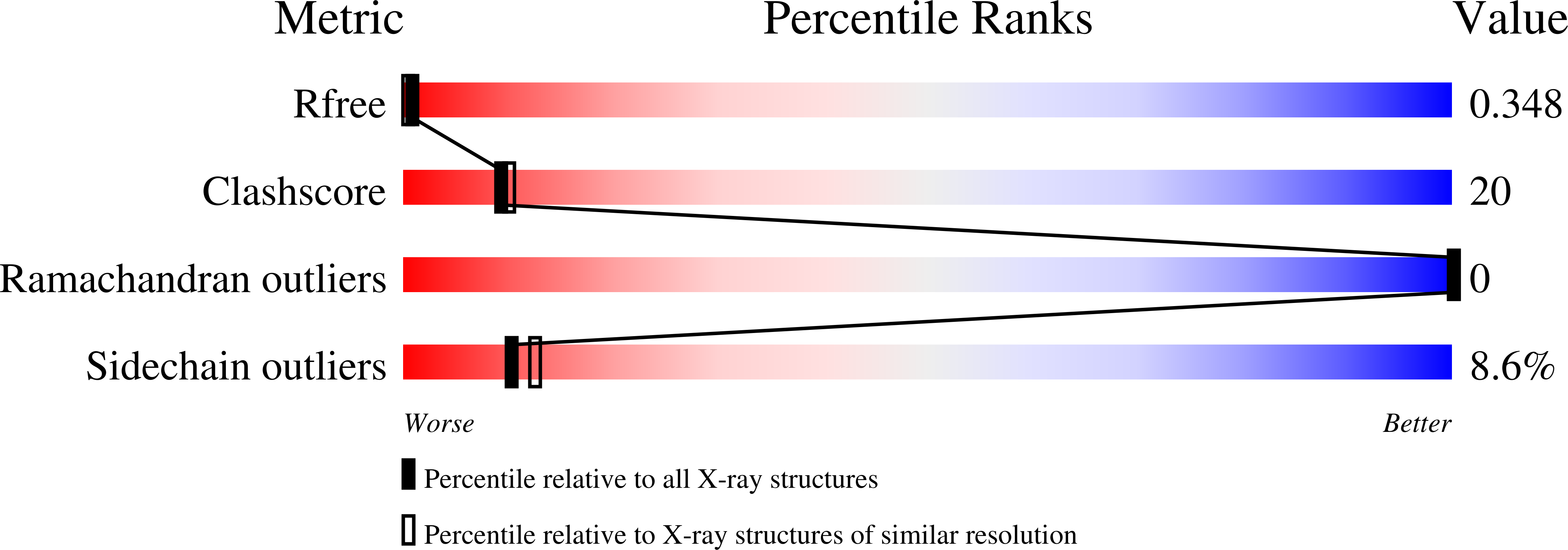Discovery of MK-4688 : an Efficient Inhibitor of the HDM2-p53 Protein-Protein Interaction.
Reutershan, M.H., Machacek, M.R., Altman, M.D., Bogen, S., Cai, M., Cammarano, C., Chen, D., Christopher, M., Cryan, J., Daublain, P., Fradera, X., Geda, P., Goldenblatt, P., Hill, A.D., Kemper, R.A., Kutilek, V., Li, C., Martinez, M., McCoy, M., Nair, L., Pan, W., Thompson, C.F., Scapin, G., Shizuka, M., Spatz, M.L., Steinhuebel, D., Sun, B., Voss, M.E., Wang, X., Yang, L., Yeh, T.C., Dussault, I., Marshall, C.G., Trotter, B.W.(2021) J Med Chem 64: 16213-16241
- PubMed: 34714078
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01524
- Primary Citation of Related Structures:
7NA1, 7NA2, 7NA3, 7NA4 - PubMed Abstract:
Identification of low-dose, low-molecular-weight, drug-like inhibitors of protein-protein interactions (PPIs) is a challenging area of research. Despite the challenges, the therapeutic potential of PPI inhibition has driven significant efforts toward this goal. Adding to recent success in this area, we describe herein our efforts to optimize a novel purine carboxylic acid-derived inhibitor of the HDM2-p53 PPI into a series of low-projected dose inhibitors with overall favorable pharmacokinetic and physical properties. Ultimately, a strategy focused on leveraging known binding hot spots coupled with biostructural information to guide the design of conformationally constrained analogs and a focus on efficiency metrics led to the discovery of MK-4688 (compound 56 ), a highly potent, selective, and low-molecular-weight inhibitor suitable for clinical investigation.
Organizational Affiliation:
Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, Massachusetts 02115, United States.