Design and in vitro characterization of RXR variants as tools to investigate the biological role of endogenous rexinoids.
le Maire, A., Rey, M., Vivat, V., Guee, L., Blanc, P., Malosse, C., Chamot-Rooke, J., Germain, P., Bourguet, W.(2022) J Mol Endocrinol 69: 377-390
- PubMed: 35900852
- DOI: https://doi.org/10.1530/JME-22-0021
- Primary Citation of Related Structures:
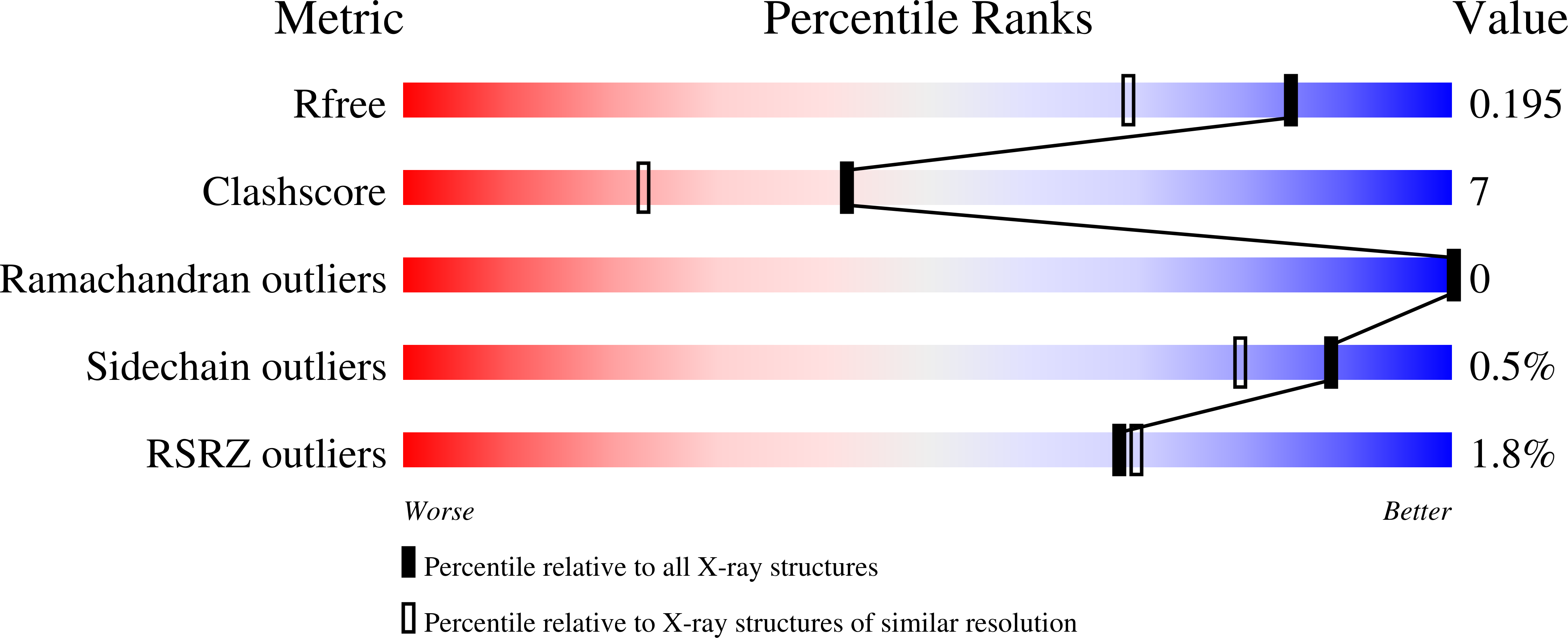
7PDQ, 7PDT, 7QAA - PubMed Abstract:
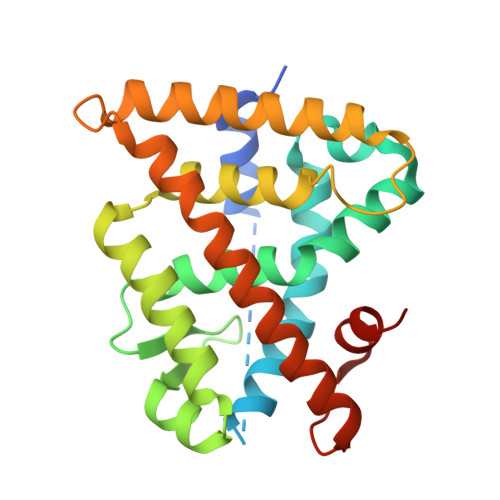
Retinoid X receptors (RXRα, β, and γ) are essential members of the nuclear receptor (NR) superfamily of ligand-dependent transcriptional regulators that bind DNA response elements and control the expression of large gene networks. As obligate heterodimerization partners of many NRs, RXRs are involved in a variety of pathophysiological processes. However, despite this central role in NR signaling, there is still no consensus regarding the precise biological functions of RXRs and the putative role of the endogenous ligands (rexinoids) previously proposed for these receptors. Based on available crystal structures, we introduced a series of amino acid substitutions into the ligand-binding pocket of all three RXR subtypes in order to alter their binding properties. Subsequent characterization using a battery of cell-based and in vitro assays led to the identification of a double mutation abolishing the binding of any ligand while keeping the other receptor functions intact and a triple mutation that selectively impairs interaction with natural rexinoids but not with some synthetic ligands. We also report crystal structures that help understand the specific ligand-binding capabilities of both variants. These RXR variants, either fully disabled for ligand binding or retaining the property of being activated by synthetic compounds, represent unique tools that could be used in future studies to probe the presence of active endogenous rexinoids in tissues/organs and to investigate their role in vivo. Last, we provide data suggesting a possible involvement of fatty acids in the weak interaction of RXRs with corepressors.
Organizational Affiliation:
CBS (Centre de Biologie Structurale), Univ Montpellier, CNRS, Inserm, Montpellier, France.