A systematic exploration of boceprevir-based main protease inhibitors as SARS-CoV-2 antivirals.
Alugubelli, Y.R., Geng, Z.Z., Yang, K.S., Shaabani, N., Khatua, K., Ma, X.R., Vatansever, E.C., Cho, C.C., Ma, Y., Xiao, J., Blankenship, L.R., Yu, G., Sankaran, B., Li, P., Allen, R., Ji, H., Xu, S., Liu, W.R.(2022) Eur J Med Chem 240: 114596-114596
- PubMed: 35839690
- DOI: https://doi.org/10.1016/j.ejmech.2022.114596
- Primary Citation of Related Structures:
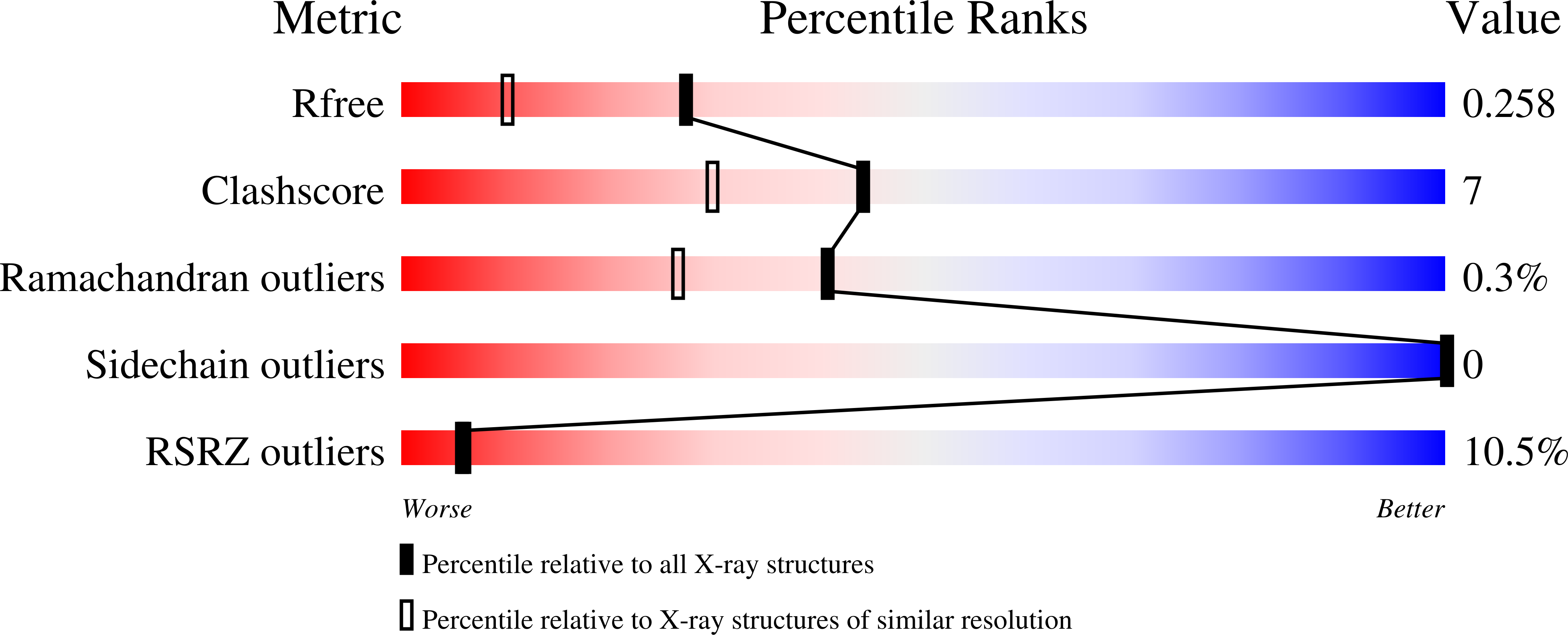
7S6W, 7S6X, 7S6Y, 7S6Z, 7S70, 7S71, 7S72, 7S73, 7S74, 7S75 - PubMed Abstract:
Boceprevir is an HCV NSP3 inhibitor that was explored as a repurposed drug for COVID-19. It inhibits the SARS-CoV-2 main protease (M Pro ) and contains an α-ketoamide warhead, a P1 β-cyclobutylalanyl moiety, a P2 dimethylcyclopropylproline, a P3 tert-butylglycine, and a P4 N-terminal tert-butylcarbamide. By introducing modifications at all four positions, we synthesized 20 boceprevir-based M Pro inhibitors including PF-07321332 and characterized their M Pro inhibition potency in test tubes (in vitro) and 293T cells (in cellulo). Crystal structures of M Pro bound with 10 inhibitors and cytotoxicity and antiviral potency of 4 inhibitors were characterized as well. Replacing the P1 site with a β-(S-2-oxopyrrolidin-3-yl)-alanyl (Opal) residue and the warhead with an aldehyde leads to high in vitro potency. The original moieties at P2, P3 and the P4 N-terminal cap positions in boceprevir are better than other tested chemical moieties for high in vitro potency. In crystal structures, all inhibitors form a covalent adduct with the M Pro active site cysteine. The P1 Opal residue, P2 dimethylcyclopropylproline and P4 N-terminal tert-butylcarbamide make strong hydrophobic interactions with M Pro , explaining high in vitro potency of inhibitors that contain these moieties. A unique observation was made with an inhibitor that contains a P4 N-terminal isovaleramide. In its M Pro complex structure, the P4 N-terminal isovaleramide is tucked deep in a small pocket of M Pro that originally recognizes a P4 alanine side chain in a substrate. Although all inhibitors show high in vitro potency, they have drastically different in cellulo potency to inhibit ectopically expressed M Pro in human 293T cells. In general, inhibitors with a P4 N-terminal carbamide or amide have low in cellulo potency. This trend is reversed when the P4 N-terminal cap is changed to a carbamate. The installation of a P3 O-tert-butyl-threonine improves in cellulo potency. Three molecules that contain a P4 N-terminal carbamate were advanced to cytotoxicity tests on 293T cells and antiviral potency tests on three SARS-CoV-2 variants. They all have relatively low cytotoxicity and high antiviral potency with EC 50 values around 1 μM. A control compound with a nitrile warhead and a P4 N-terminal amide has undetectable antiviral potency. Based on all observations, we conclude that a P4 N-terminal carbamate in a boceprevir derivative is key for high antiviral potency against SARS-CoV-2.
Organizational Affiliation:
Texas A&M Drug Discovery Laboratory, Department of Chemistry, Texas A&M University, College Station, TX, 77843, USA.