Discovery and Structure-Based Design of Potent Covalent PPAR gamma Inverse-Agonists BAY-4931 and BAY-0069 .
Orsi, D.L., Pook, E., Brauer, N., Friberg, A., Lienau, P., Lemke, C.T., Stellfeld, T., Bruggemeier, U., Putter, V., Meyer, H., Baco, M., Tang, S., Cherniack, A.D., Westlake, L., Bender, S.A., Kocak, M., Strathdee, C.A., Meyerson, M., Eis, K., Goldstein, J.T.(2022) J Med Chem 65: 14843-14863
- PubMed: 36270630
- DOI: https://doi.org/10.1021/acs.jmedchem.2c01379
- Primary Citation of Related Structures:
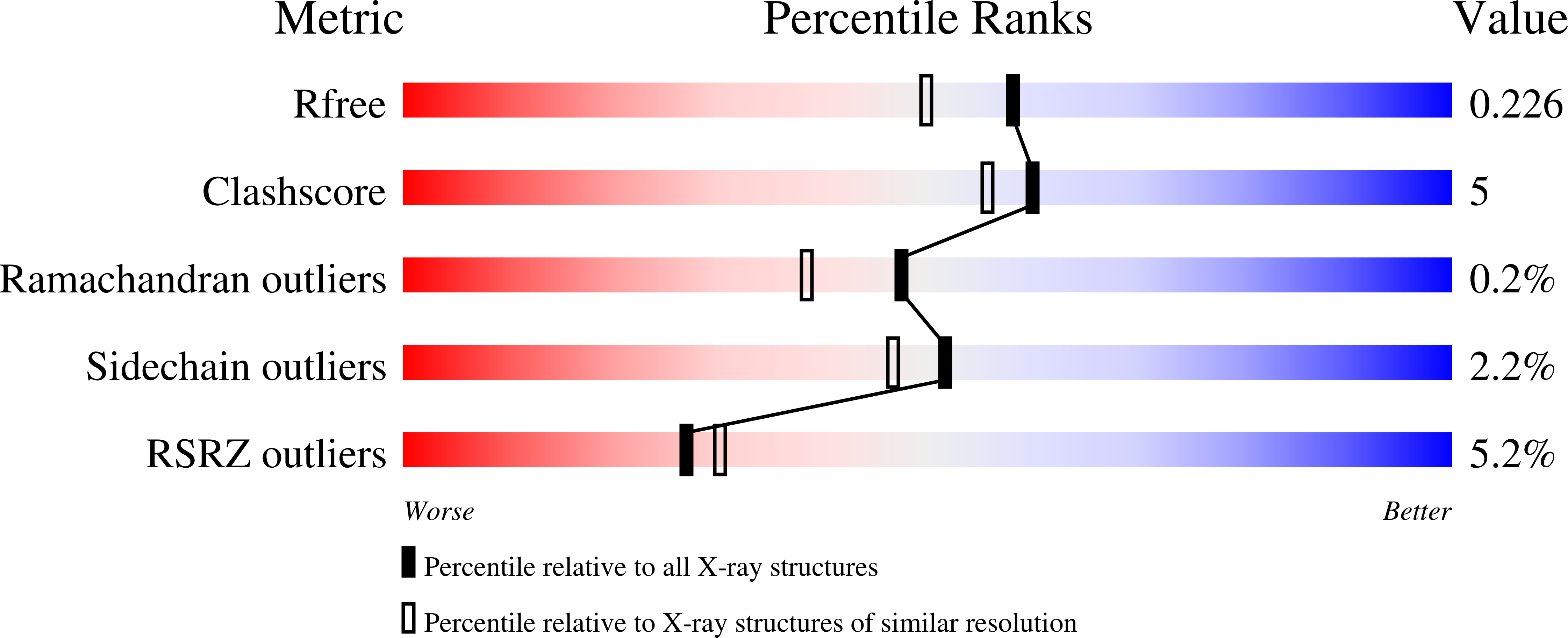
8AQM, 8AQN - PubMed Abstract:
The ligand-activated nuclear receptor peroxisome-proliferator-activated receptor-γ (PPARG or PPARγ) represents a potential target for a new generation of cancer therapeutics, especially in muscle-invasive luminal bladder cancer where PPARγ is a critical lineage driver. Here we disclose the discovery of a series of chloro-nitro-arene covalent inverse-agonists of PPARγ that exploit a benzoxazole core to improve interactions with corepressors NCOR1 and NCOR2. In vitro treatment of sensitive cell lines with these compounds results in the robust regulation of PPARγ target genes and antiproliferative effects. Despite their imperfect physicochemical properties, the compounds showed modest pharmacodynamic target regulation in vivo . Improvements to the in vitro potency and efficacy of BAY-4931 and BAY-0069 compared to those of previously described PPARγ inverse-agonists show that these compounds are novel tools for probing the in vitro biology of PPARγ inverse-agonism.
Organizational Affiliation:
Center for the Development of Therapeutics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142, United States.