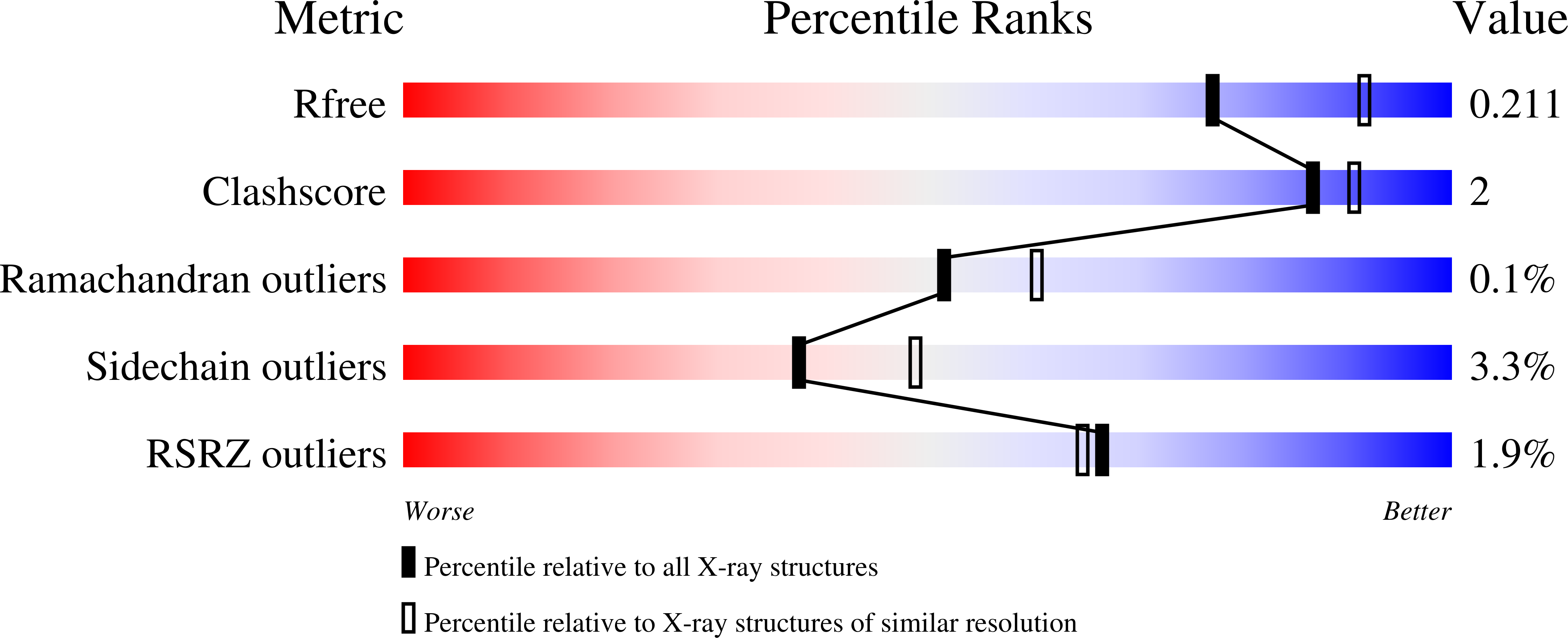
Structural and dynamic effects of paraoxon binding to human acetylcholinesterase by X-ray crystallography and inelastic neutron scattering.
Gerlits, O., Fajer, M., Cheng, X., Blumenthal, D.K., Radic, Z., Kovalevsky, A.(2022) Structure 30: 1538-1549.e3
- PubMed: 36265484
- DOI: https://doi.org/10.1016/j.str.2022.09.006
- Primary Citation of Related Structures:
8DT2, 8DT4, 8DT5, 8DT7 - PubMed Abstract:
Organophosphorus (OP) compounds, including nerve agents and some pesticides, covalently bind to the catalytic serine of human acetylcholinesterase (hAChE), thereby inhibiting acetylcholine hydrolysis necessary for efficient neurotransmission. Oxime antidotes can reactivate the OP-conjugated hAChE, but reactivation efficiency can be low for pesticides, such as paraoxon (POX). Understanding structural and dynamic determinants of OP inhibition and reactivation can provide insights to design improved reactivators. Here, X-ray structures of hAChE with unaged POX, with POX and oximes MMB4 and RS170B, and with MMB4 are reported. A significant conformational distortion of the acyl loop was observed upon POX binding, being partially restored to the native conformation by oximes. Neutron vibrational spectroscopy combined with molecular dynamics simulations showed that picosecond vibrational dynamics of the acyl loop soften in the ∼20-50 cm -1 frequency range. The acyl loop structural perturbations may be correlated with its picosecond vibrational dynamics to yield more comprehensive template for structure-based reactivator design.
Organizational Affiliation:
Department of Natural Sciences, Tennessee Wesleyan University, Athens, TN 37303, USA.