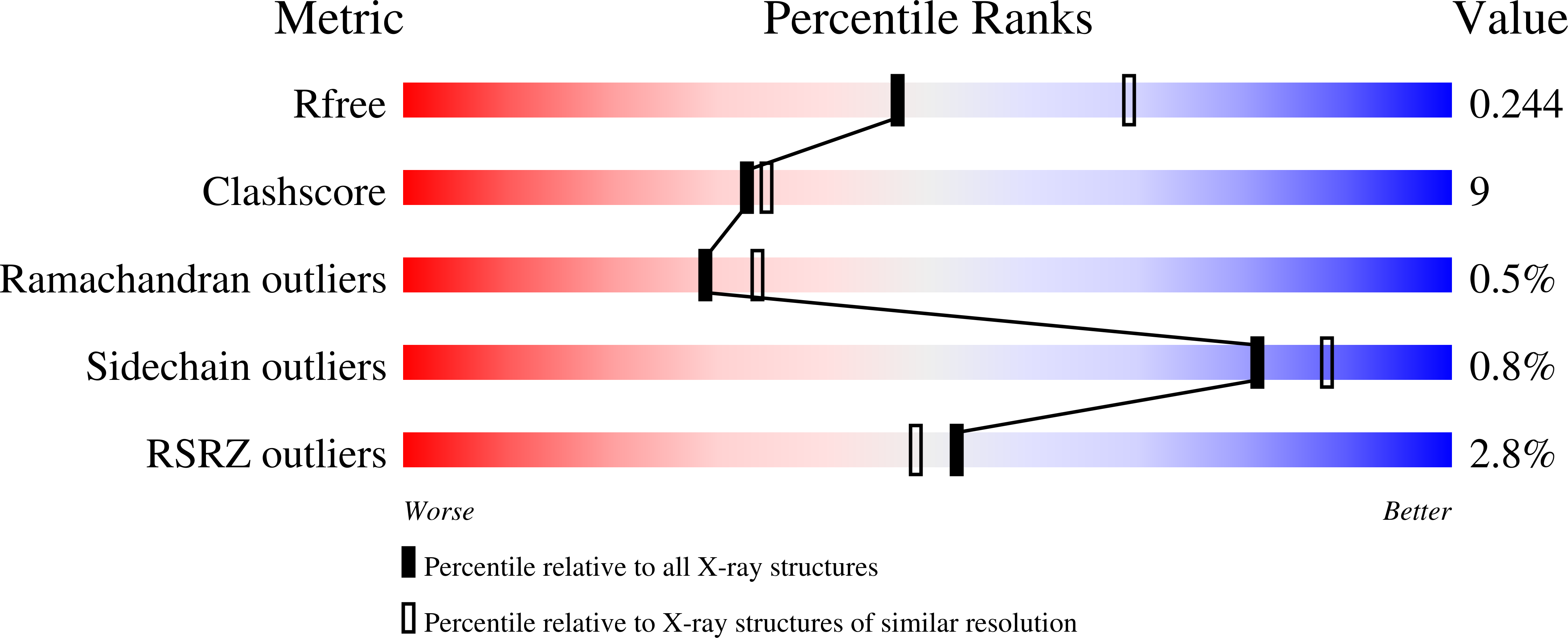
Enhanced active-site electric field accelerates enzyme catalysis.
Zheng, C., Ji, Z., Mathews, I.I., Boxer, S.G.(2023) Nat Chem 15: 1715-1721
- PubMed: 37563323
- DOI: https://doi.org/10.1038/s41557-023-01287-x
- Primary Citation of Related Structures:
7U9N, 7UQ9, 7UTW, 8EIW, 8EIX, 8EIY - PubMed Abstract:
The design and improvement of enzymes based on physical principles remain challenging. Here we demonstrate that the principle of electrostatic catalysis can be leveraged to substantially improve a natural enzyme's activity. We enhanced the active-site electric field in horse liver alcohol dehydrogenase by replacing the serine hydrogen-bond donor with threonine and replacing the catalytic Zn 2+ with Co 2+ . Based on the electric field enhancement, we make a quantitative prediction of rate acceleration-50-fold faster than the wild-type enzyme-which was in close agreement with experimental measurements. The effects of the hydrogen bonding and metal coordination, two distinct chemical forces, are described by a unified physical quantity-electric field, which is quantitative, and shown here to be additive and predictive. These results suggest a new design paradigm for both biological and non-biological catalysts.
Organizational Affiliation:
Department of Chemistry, Stanford University, Stanford, CA, USA.